In this issue of a Blood, in a first-rate example of collaboration between academia (University of California) and industry (Sangamo), Hoban et al show in situ gene correction of sickle cell anemia (SCA), a prototypical hemoglobinopathy.1
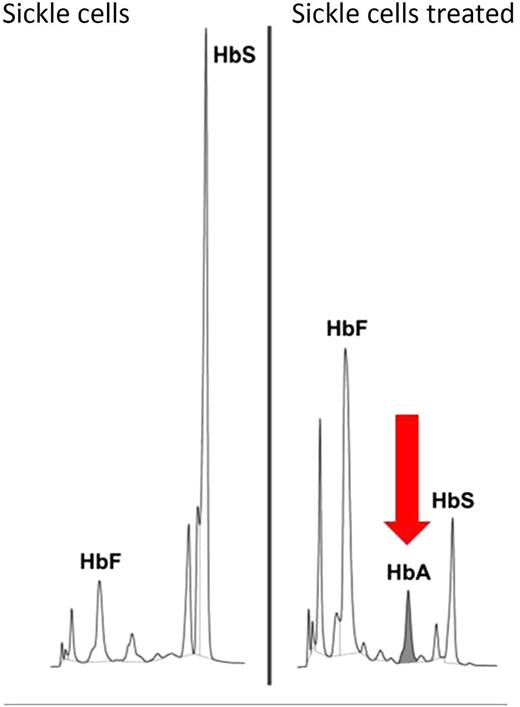
The peak of healthy hemoglobin A (HbA) in gene-edited human erythroid cells is the evidence of its functional restoration from the mutated β-globin S allele. HbS, sickle cell hemoglobin; HbF, fetal hemoglobin. Adapted from Figure 5B in the article by Hoban et al that begins on page 2597.
The peak of healthy hemoglobin A (HbA) in gene-edited human erythroid cells is the evidence of its functional restoration from the mutated β-globin S allele. HbS, sickle cell hemoglobin; HbF, fetal hemoglobin. Adapted from Figure 5B in the article by Hoban et al that begins on page 2597.
The mechanism of SCA as a molecular disease was first demonstrated by Linus Pauling, who showed that the abnormal hemoglobin of SCA had a different electrophoretic mobility from cells than normal hemoglobin and, in the process, suggesting that a change in the sequence or makeup of amino acids caused the disease. Using the tools of chemistry to approach the problem of SCA was a major conceptual shift from a descriptive, almost botanical, model of human pathology, and was an early affirmation that basic science was to be our most successful method for understanding disease.2 Next in this history, Vernon Ingram confirmed that substitution of a single amino acid (glutamate to valine in the case of SCA) in a protein can result in a human disease.3 Building on this idea led Francis Crick and Sydney Brenner to one of the most influential insights in all of modern biology: that triplets of DNA bases determine the amino acid sequence of proteins.4 Without these critical steps, the field of molecular biology might not exist. Thus, a single DNA mutation (such as adenine to thymine transversion in β-globin) can result in clinical disorder. In this fashion, the scientific history of SCA has made it both amenable to the principles of design and construction of gene tools, as well as a highly desirable target for correction of this first genetic disease by genetic means.
Hoban et al merge the technology of gene editing using zinc finger nucleases (ZFNs) with their expertise in delivering these designer molecules into a relevant cell type: hematopoietic stem cells (HSCs). Critically, in the light of current discussion, HSCs are somatic—not germline—cells. They show proof of concept for restoration of hemoglobin gene function in the context of its physiological regulatory elements (that is, promoter and locus control region) and also refine our understanding of the biological mechanisms underlying gene correction by homology-directed repair (HDR) in HSCs. This superb advance was made possible by harnessing the natural ability of a cell to repair its genome. DNA in each cell of our body experiences thousands of cuts (double-stranded breaks) every day. To survive, the cell needs to repair itself. It has 2 major options: (1) glue the frayed DNA ends together, which typically happens with a resulting addition or loss of DNA parts (termed nonhomologous end joining [NHEJ]); or (2) seek a DNA template (eg, a sister chromatid) that guides perfect restoration of the wild-type DNA to function by HDR. The genius of gene editing technology is in turning this physiologic DNA repair into a therapeutic one. NHEJ has been used to disable genes (such as the HIV coreceptor C-C chemokine receptor 5) and HDR to correct them, seamlessly.
Gene editing molecules are designed to perform 2 tasks: recognize the locus of interest and cleave the DNA. Accordingly, Hoban et al deliver a pair of ZFNs capable of cutting the β-globin locus, along with a homologous wild-type oligonucleotide or with a donor template cloned into an integrase-defective lentiviral vector, into human HSCs obtained from either healthy people or from individuals with SCA. The ZFNs were highly specific. Approximately one-fifth of erythroid progenitor cells were corrected (see figure). Off-target effects were largely confined to the homologous and functionally dispensable δ-globin gene. Of critical importance for future improvements, these studies showed that gene correction is far less efficient in HSCs than in hematopoietic progenitor cells, likely due to a bias toward NHEJ in HSCs and toward HDR in the more mature hematopoietic cells.
The clinical future of this effort will be determined by the next steps. First, it will be critical to make this technology validated, robust, and simplified enough to be acceptable for clinical development.5,6 This is where the fact that the academia-industry interface has been present in this work since its inception can prove extremely useful. Second, this iterative process will benefit from the methodological advances of others, for example, recent use of ZFNs for integration of corrective DNA into the interleukin 2 receptor γ gene in HSCs from an individual with X-linked severe combined immunodeficiency.7 Third, gene therapy technology needs to be developed simultaneously with protocols to sustain and maintain autologous HSCs through the collection and transgenesis steps and with a clinical transplantation trial to mediate their significant and permanent engraftment.8
Hematopoietic cell transplantation (HCT) was the first gene therapy.9 It has been used as a life-saving measure for multiple genetic disorders (in some individuals with SCA, for example, or in mucopolysaccharidosis type I, Fanconi anemia, dyskeratosis congenita, genetic forms of immune deficiencies, adrenoleukodystrophy, epidermolysis bullosa, etc). Viral-mediated gene therapy is already in clinical trials (or in preclinical development) for a majority of them. Gene editing has the potential to be the next conceptual step in gene therapy. Critically, even partial gene correction is likely to be clinically meaningful, as SCA heterozygotes are typically free of disease symptoms, and recipients of HCT with mixed chimerism can derive significant clinical benefits from as little as one-fifth donor hematopoietic cells.
With a quarter of a million new cases each year, SCA is a tremendous health care challenge worldwide. It results in massive human suffering, from pain caused by capricious and sometimes intractable vaso-occlusive or sequestration crises to the chronic stress of dealing with infections, chronic hemolysis, and progressive multiorgan system complications. The current hope is that editing out the one SCA-causing genomic misprint—along with other treatment measures—will relieve those living with it from numerous sources of pain, among them spleen sequestration. Xavier Bichat said that human disease is “a revolt of organs,” and health is “their silence.”10 If this is true, gene editing may give SCA sufferers symptom-free internal organs, including a “silent spleen,” and, by removing the cause of the disease, give them fuller, longer, more complete lives.
Conflict-of-interest disclosure: The author declares no competing financial interests.