In this issue of Blood, Savona and an international consortium of clinical investigators propose uniform response criteria for treatment trials enrolling adult patients with myelodysplastic/myeloproliferative neoplasms (MDS/MPNs).1 Such a proposal is needed because new drugs are finally being tested in these rare “overlap” syndromes that have both dysplastic and proliferative pathological features, and neither the International Working Group (IWG) response criteria for myelodysplastic syndromes2 nor the IWG Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) response criteria for myelofibrosis3 or for other myeloproliferative neoplasms fit such patients well.
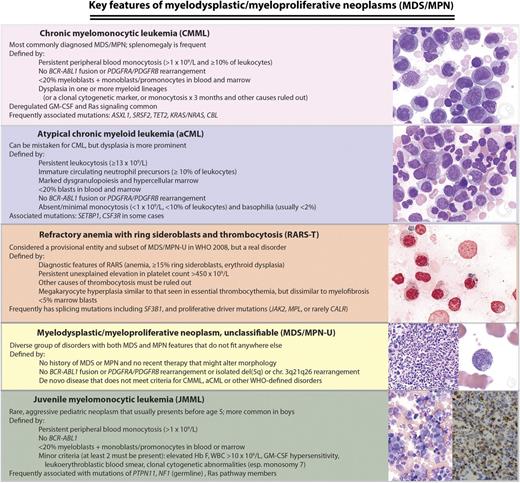
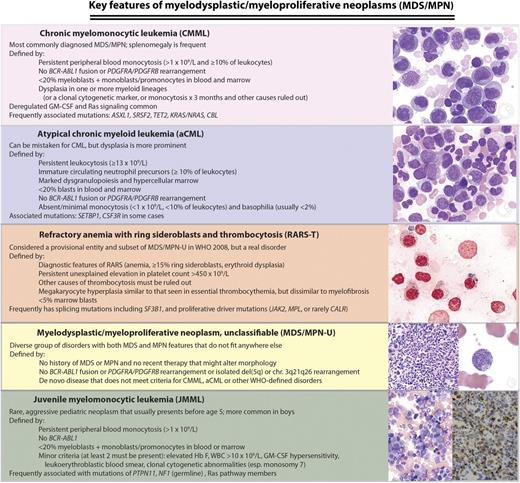
WHO (2008) classification of MDS/MPNs: disease definitions and key molecular features. Chronic myelomonocytic leukemia (CMML), atypical chronic myeloid leukemia (aCML), MDS/MPN-U, and left juvenile myelomonocytic leukemia (JMML) micrographs are Wright-Giemsa stained marrow aspirate. Right JMML micrograph is a CD68 immunostain highlighting neoplastic marrow monocytes, and refractory anemia with ring sideroblasts and thrombocytosis (RARS-T) image is a Prussian blue reaction demonstrating numerous ring sideroblasts. Microscopy conditions are described at http:imagebank.hematology.org. GM-CSF, granulocyte-macrophage colony stimulating factor; Hb F, fetal hemoglobin; MPN-U, unclassifiable MDS/MPN; WBC, white blood count; WHO, World Health Organization. Image source: ASH Image Bank (© American Society of Hematology); images (top to bottom) 2149, 2224, 2627, 4031, 2119, 1095, and 1098.
WHO (2008) classification of MDS/MPNs: disease definitions and key molecular features. Chronic myelomonocytic leukemia (CMML), atypical chronic myeloid leukemia (aCML), MDS/MPN-U, and left juvenile myelomonocytic leukemia (JMML) micrographs are Wright-Giemsa stained marrow aspirate. Right JMML micrograph is a CD68 immunostain highlighting neoplastic marrow monocytes, and refractory anemia with ring sideroblasts and thrombocytosis (RARS-T) image is a Prussian blue reaction demonstrating numerous ring sideroblasts. Microscopy conditions are described at http:imagebank.hematology.org. GM-CSF, granulocyte-macrophage colony stimulating factor; Hb F, fetal hemoglobin; MPN-U, unclassifiable MDS/MPN; WBC, white blood count; WHO, World Health Organization. Image source: ASH Image Bank (© American Society of Hematology); images (top to bottom) 2149, 2224, 2627, 4031, 2119, 1095, and 1098.
MDS/MPNs are clinically heterogeneous and biologically poorly understood, although some pathophysiological insights have begun to emerge from high-throughput genetic analyses and murine models.4 Four somewhat distinct clinicopathological syndromes (see figure) are currently recognized by the World Health Organization (WHO)5 : CMML (the most common syndrome in the group), JMML (an aggressive pediatric disease), aCML, and RARS-T (currently a “provisional” entity that will lose its provisional nature in the next WHO classification iteration). Rare patients who exhibit both cellular dysplasia and myeloproliferative features but do not meet criteria for any of the specific syndromes are considered to have unclassifiable MDS/MPN, a diverse group with a highly variable clinical course.6
For the most part, because patients with MDS/MPNs other than JMML tend to be elderly, and outcomes with allogeneic hematopoietic stem cell transplant in this group of neoplasms are notoriously poor, patients diagnosed with MDS/MPNs are treated primarily with palliative, supportive measures. Extramedullary hematopoiesis, leukocytosis, and thrombocytosis in MDS/MPNs are often approached with hydroxyurea or other cytoreductive agents, whereas hematopoietic growth factors, androgens, corticosteroids, and other drugs are commonly employed as adjuncts to transfusions to ameliorate cytopenias. Occasionally, a hypomethylating agent such as azacitidine or an immunomodulatory drug such as lenalidomide can induce simultaneous improvement in both dysplastic and proliferative disease features, but such doubly good outcomes are infrequent, and disease-associated symptoms may persist even when blood counts improve.
Patients with MDS/MPNs rarely have specific molecular abnormalities targetable with currently available agents, which highlights the potential for improved approaches to these disorders in the near future. For instance, the recent finding of recurrent CSF3R mutations in aCML7 has prompted the use of dasatinib or ruxolitinib, depending on the specific CSF3R domain mutated; in our practice at Dana-Farber Cancer Institute, a number of patients with aCML have experienced favorable responses to these drugs. Because of the critical role of GM-CSF dysregulation and downstream JAK-STAT signaling in CMML biology, antibodies against GM-CSF and inhibitors of JAK2, including ruxolitinib, are currently being evaluated clinically in CMML.8 RARS-T is commonly associated with both spliceosome mutations, especially SF3B1, as well as mutations associated with cytokine signaling and myeloproliferation (eg, JAK2, MPL, CALR), but this entity is so rare that disease-specific clinical trials have proven difficult to conduct. However, anecdotes of favorable response to lenalidomide in RARS-T, even in the absence of del(5q), should prompt further investigation of the mechanism of response.9
To evaluate new therapies systematically, uniform response criteria are required. Consensus criteria to describe the response to nontransplant therapies in JMML have recently been published,10 but to date there have been no criteria for adult MDS/MPNs. MDS response criteria fail to capture the whole picture when, for example, a drug shrinks the spleen, yet anemia worsens. Is that a beneficial response or evidence of progressive disease? And although MPN response criteria address both cytopenias and organomegaly, improved quality of life and possibly even survival with ruxolitinib use in patients who lack objective responses meeting IWG-MRT criteria highlight some of the limitations of response measures for such complex disorders.
The current proposal for MDS/MPNs by Savona and colleagues1 recognizes that “clinical benefit” comes in many forms. These new criteria emerged from 3 workshops in which candidate measures were proposed, discussed, ranked, and revised. The authors tried to keep the new criteria as similar to familiar MDS and MPN response categories as possible, while recognizing the unique constellation of signs and symptoms faced by patients with MDS/MPNs. Importantly, the new criteria underscore the critical importance of patient symptoms, and the authors even propose 2 variants of “complete response”: with and without residual symptoms, measured with tools such as the familiar Myelofibrosis Symptom Assessment Form.11 The origin of certain symptoms may be difficult to ascribe to MDS/MPNs vs another medical disorder (such as fatigue in some patients with comorbid conditions), which may cause practical problems in assigning a symptom response to the experimental therapy.
As with any new proposal, these criteria need to be prospectively validated, and only their use in the real-world clinical trial settings will demonstrate their value. As with many “consensus” guidelines and criteria, it is unclear how panel members were chosen beyond being a coalition of the willing, because there was no formal call for applications, and several active investigators in MDS/MPNs are missing from the author list. It is hoped that this omission will not impede the use and adoption of these criteria. A different group of investigators might have come up with slightly different criteria, but the chosen criteria address the most common patient complaints and most widely assessed disease markers in contemporary clinical practice.
Ultimately, response criteria are clinical Esperanto: communication tools that facilitate comparison of treatment approaches and that make it easier for investigators to write therapeutic protocols, because home-brew response criteria no longer have to be generated for every study. Like all conversations, this one will evolve over time, but someone had to speak first.
Conflict-of-interest disclosure: The author declares no competing financial interests.