Key Points
SLAP and SLAP2 have redundant functions in the regulation of platelet (hem)ITAM signaling.
SLAP and SLAP2 in platelets limit occlusive thrombus formation and ischemic brain infarction.
Abstract
Glycoprotein VI and C-type lectin-like receptor 2 are essential platelet activating receptors in hemostasis and thrombo-inflammatory disease, which signal through a (hem)immunoreceptor tyrosine-based activation motif (ITAM)-dependent pathway. The adapter molecules Src-like adapter proteins (SLAP and SLAP2) are involved in the regulation of immune cell surface expression and signaling, but their function in platelets is unknown. In this study, we show that platelets expressed both SLAP isoforms and that overexpression of either protein in a heterologous cell line almost completely inhibited glycoprotein VI and C-type lectin-like receptor 2 signaling. In mice, single deficiency of SLAP or SLAP2 had only moderate effects on platelet function, whereas double deficiency of both adapters resulted in markedly increased signal transduction, integrin activation, granule release, aggregation, procoagulant activity, and thrombin generation in response to (hem)ITAM-coupled, but not G protein-coupled, receptor activation. In vivo, constitutive SLAP/SLAP2 knockout mice displayed accelerated occlusive arterial thrombus formation and a dramatically worsened outcome after focal cerebral ischemia. This was attributed to the absence of both adapter proteins in platelets, as demonstrated by adoptive transfer of Slap−/−/Slap2−/− platelets into wild-type mice. Our results establish SLAP and SLAP2 as critical inhibitors of platelet (hem)ITAM signaling in the setting of arterial thrombosis and ischemic stroke.
Introduction
Platelet activation at sites of vascular injury is essential for hemostasis, but is also a major pathomechanism underlying myocardial infarction and stroke.1,2 The central platelet collagen receptor glycoprotein (GP) VI/FcRγ-chain complex3,4 critically contributes to this activation, and its loss or functional inhibition provides profound antithrombotic protection, but only moderately increased bleeding in vivo.4,5
GPVI signals through an immunoreceptor tyrosine-based activation motif (ITAM) pathway in a similar manner to the T- and B-cell antigen receptors (TCR, BCR) and some Fc receptors. Ligand-induced crosslinking of GPVI leads to phosphorylation of the two tyrosine residues within the ITAM on the FcRγ-chain predominantly by the Src family kinase (SFK) Lyn,6,7 followed by the recruitment, phosphorylation, and activation of the tyrosine kinase Syk, which initiates a downstream signaling cascade ultimately resulting in the activation of effector enzymes, including phosphoinositol-3-kinases and phospholipase C (PLC) γ2.8 These signaling events downstream of Syk also occur upon stimulation of the platelet C-type lectin-like receptor 2 (CLEC-2) either by its endogenous ligand, the transmembrane GP podoplanin, or by the snake venom toxin rhodocytin. CLEC-2 is an ∼30 kilodalton (kDa) type II membrane protein that contains a single conserved cytosolic YXXL sequence (hemITAM) which initiates signaling upon CLEC-2 dimerization or oligomerization.8 CLEC-2 is highly expressed on megakaryocytes and platelets and at lower levels on a number of leukocytes.9-11 CLEC-2 has been identified as a critical player in a plethora of (patho-)physiological processes, including thrombus formation and stability, lymphatic development, and tumor metastasis, and similar to GPVI, in the maintenance of vascular integrity during inflammation.10,12,13
Src-like adapter proteins (SLAP and SLAP2) constitute a family of adapter molecules of 34 kDa and 25/28 kDa, respectively, that share structural similarities with SFKs, characterized by the presence of a unique N-terminal region, an SH3-, and an SH2-domain.14 Unlike SFKs, SLAP and SLAP2 do not possess a C-terminal kinase domain.14 Overexpression studies in T- and B-cell lines indicated that SLAP and SLAP2 act as negative regulators of TCR and BCR signaling,15-19 and contribute to TCR and BCR surface expression levels.18-21 The latter mechanism involves the interaction of SLAP proteins with phosphorylated components of the TCR or BCR complex, followed by the recruitment of the E3 ubiquitin ligase c-Cbl to the receptor complex which promotes its degradation.19,21,22 In this way, SLAP plays an important role in the regulation of T- and B-cell maturation and development.18,20
Little is known about the function of SLAP proteins in platelets, other than upon platelet stimulation specifically with the GPVI-activating snake venom protein convulxin, SLAP2 co-immunoprecipitates with c-Cbl, Syk, and LAT.23 The functional consequences of these putative interactions are unknown and form the aim of this study.
Methods
Animals
Slap−/−, Slap2−/−, and Slap−/−/Slap2−/− mice were generated as previously described,20,24 and these mice were backcrossed for 10 generations onto the BALB/c background. Gp6−/− mice13 were crossed with Slap−/−/Slap2−/− mice. Slap−/−/Slap2−/−/Gp6+/− and litter-matched Slap−/−/Slap2−/−/Gp6+/+ mice on a mixed BALB/c/Sv129/C57BL/6 background were used in this study. Animal studies conducted were approved by the district government of Lower Frankonia (Bezirksregierung Unterfranken, Germany).
Transfections and luciferase assays
The DT40 B-cell line was transfected by a previously published electroporation method,25 which is described in detail in the supplemental Methods on the Blood Web site.
In vitro platelet studies
Immunoprecipitation is described in the supplemental Methods. Platelet preparation, western blot analysis, aggregometry, flow cytometry, adenosine 5′-triphosphate (ATP) release, quantification of phosphatidylserine (PS) exposure, and thrombin generation were performed as described previously.26-28
Tail bleeding time
Mice were anesthetized, 1 mm of the tail tip was removed with a scalpel, and the tails were immersed in 0.9% isotonic saline (37°C). The time until cessation of bleeding (no blood flow for 1 minute) was determined.
Mechanical injury of the abdominal aorta
An ultrasonic flow probe (0.5PSB699; Transonic Systems) was placed around the abdominal aorta of anesthetized mice and thrombus formation was induced by a single firm compression with a forceps for 10 seconds. Blood flow was monitored until complete blood vessel occlusion occurred for at least 5 minutes, or for a maximum of 30 minutes.
Thrombus formation in FeCl3-injured carotid arteries
An ultrasonic flow probe (0.5PSB699; Transonic Systems) was placed around the exposed carotid artery of anesthetized mice and thrombosis was induced by topical application of 2.5% FeCl3 for 90 seconds. For animals subjected to FeCl3–induced injury of the carotid artery after adoptive platelet transfer, 7.5% FeCl3 for 1 minute was used. Blood flow was monitored until complete blood vessel occlusion occurred for at least 2 minutes, or for a maximum of 30 minutes.
Transient middle cerebral artery (MCA) occlusion model
Focal cerebral ischemia was induced in wild-type (WT) and Slap−/−/Slap2−/− mice by a transient MCA occlusion (tMCAO) as described.29 Briefly, a silicon-coated thread was advanced through the carotid artery up to the origin of the MCA causing an MCA infarction. After an occlusion time of 30 minutes, the filament was removed allowing reperfusion of the MCA territory. The extent of infarction was quantitatively assessed 24 hours after reperfusion on 2,3,5-triphenyltetrazolium chloride stained brain sections. Global neurologic function and motor function were evaluated by the Bederson score30 and the grip test,31 respectively. The percentage of ipsilesional occluded vessels 24 hours after tMCAO was determined on hematoxylin and eosin stained brain section as previously described.32
Platelet depletion
Thrombocytopenia was induced in BALB/c WT mice by IV injection of an anti-GPIbα antibody (0.15 μg/g body weight). Peripheral platelet counts were determined by flow cytometry 12 hours after platelet depletion as previously described.33
Platelet transfusion
Washed platelets from several donor mice were pooled and 109 platelets in 200 μL Tyrode’s buffer were transferred IV into WT mice. Purity of the platelet suspension was confirmed by flow cytometry and microscopical inspection, and contamination by other blood cell types was further excluded using a fully automated hematology analyzer (Sysmex KX-21N). Peripheral platelet counts were determined 30 minutes after platelet transfer, and mice were subsequently subjected to FeCl3-injury of the carotid artery, or 30 minutes of tMCAO.
Chemicals and data analysis
A list of antibodies and reagents, and statistical data analysis are provided in the supplemental Methods.
Results
SLAP and SLAP2 independently prevent GPVI and CLEC-2 signaling in a cell line model without affecting surface expression levels
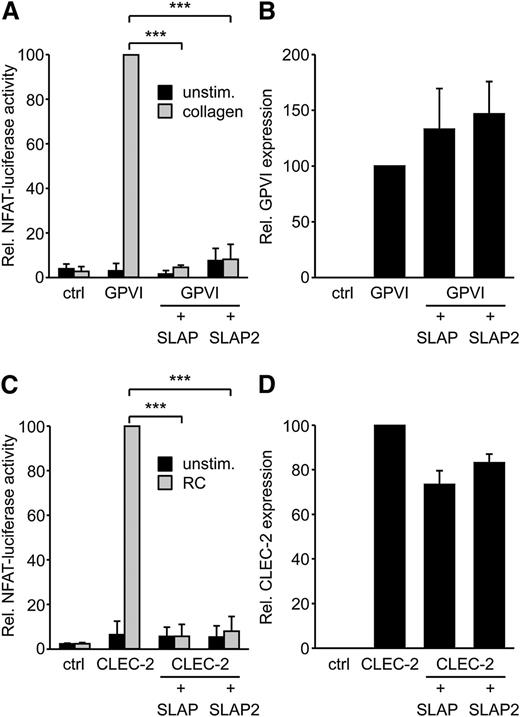
SLAP proteins have been identified as negative regulators of TCR and BCR expression levels and signaling.14 Platelet GPVI/FcRγ signaling resembles the signaling pathways downstream of the TCR and BCR complexes. Therefore, we hypothesized that SLAP and SLAP2 might have the capacity to regulate GPVI signaling. SLAP proteins were expressed in the DT40 B-cell line model system that we have previously used to study GPVI/FcRγ signaling.25 In this assay, an NFAT/AP-1 transcriptional reporter produces luciferase in response to combined Ca2+ and mitogen-activated protein kinase signaling, both of which are downstream of GPVI/FcRγ activation. Strikingly, SLAP and SLAP2 almost completely inhibited GPVI signaling in response to collagen (Figure 1A), without affecting the expression levels of transfected GPVI (Figure 1B). Cells transfected with SLAP and SLAP2 responded normally to phorbol myristate acetate and ionomycin stimulation (data not shown), indicating that the SLAP proteins were not nonspecifically inhibiting cell signaling in a global manner.
SLAP and SLAP2 inhibit GPVI/FcRγ and CLEC-2 signaling in the DT40 B-cell line model. (A) The DT40 B-cell line was transfected with a Ca2+/ mitogen-activated protein kinase-responsive NFAT/AP-1-luciferase reporter construct, a β-galactosidase construct, a GPVI, an FcRγ, a SLAP or SLAP2 expression construct, or empty vector control. Cells were either left unstimulated or stimulated with 5 μg/mL collagen, lysed, and assayed for luciferase and β-galactosidase. Luciferase data were normalized for β-galactosidase values. (B) The expression of GPVI was confirmed by flow cytometry with an anti-GPVI mAb. (C) The experiment was conducted as described in (A), with the exception that cells were transfected with a myc-tagged CLEC-2 expression construct and stimulated with 50 nM rhodocytin. (D) The experiment was performed as for (B), except that CLEC-2 was detected by an anti-myc mAb. Results in all panels are expressed as mean ± standard deviation (SD) (n = 3). ***P < .001. ctrl, control; RC, rhodocytin; unstim., unstimulated.
SLAP and SLAP2 inhibit GPVI/FcRγ and CLEC-2 signaling in the DT40 B-cell line model. (A) The DT40 B-cell line was transfected with a Ca2+/ mitogen-activated protein kinase-responsive NFAT/AP-1-luciferase reporter construct, a β-galactosidase construct, a GPVI, an FcRγ, a SLAP or SLAP2 expression construct, or empty vector control. Cells were either left unstimulated or stimulated with 5 μg/mL collagen, lysed, and assayed for luciferase and β-galactosidase. Luciferase data were normalized for β-galactosidase values. (B) The expression of GPVI was confirmed by flow cytometry with an anti-GPVI mAb. (C) The experiment was conducted as described in (A), with the exception that cells were transfected with a myc-tagged CLEC-2 expression construct and stimulated with 50 nM rhodocytin. (D) The experiment was performed as for (B), except that CLEC-2 was detected by an anti-myc mAb. Results in all panels are expressed as mean ± standard deviation (SD) (n = 3). ***P < .001. ctrl, control; RC, rhodocytin; unstim., unstimulated.
To test the possibility that SLAP or SLAP2 might also negatively regulate signaling via the hemITAM receptor CLEC-2, the NFAT/AP-1-luciferase assay in DT40 B cells was again used, since it is an effective model system to study hemITAM signaling.34 SLAP and SLAP2 each almost completely inhibited CLEC-2 signaling in response to rhodocytin (Figure 1C), without significantly reducing the expression levels of transfected CLEC-2 (Figure 1D). Responses to phorbol-12-myrisate-13-acetate and ionomycin were not affected by the SLAP proteins (data not shown). Together, these data suggested that SLAP and SLAP2 can serve as negative regulators of both ITAM and hemITAM signaling.
SLAP and SLAP2 are expressed in mouse platelets
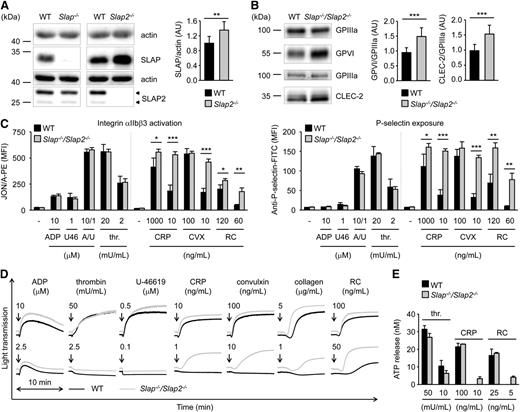
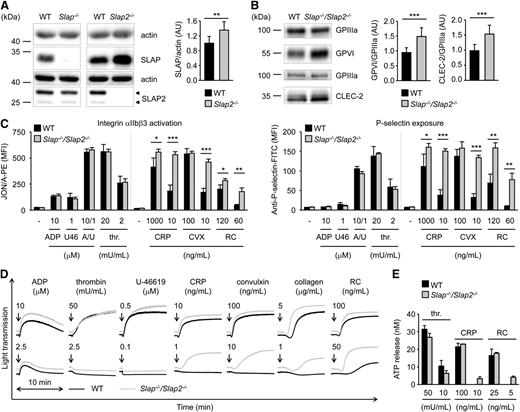
We set out to extend our data obtained in a heterologous system and to further investigate the role of SLAP family proteins in platelet biology by analyzing mice deficient for SLAP or SLAP2.20,24 Western blot analysis confirmed the absence of SLAP in Slap−/− and of SLAP2 in Slap2−/− platelet lysates (Figure 2A). We observed upregulation of SLAP in Slap2−/− platelets (increase of ∼35% compared with WT platelets) (Figure 2A). The two SLAP2 protein bands of 25 kDa and 28 kDa are in line with previous reports which indicate that alternative translation leads to the expression of two SLAP2 isoforms (Figure 2A, arrowheads).16,17 Slap−/− and Slap2−/− mice had normal platelet counts and size (supplemental Table 1). Flow cytometric measurement of prominent platelet surface receptors and western blot analysis showed slightly increased surface expression of GPVI in Slap−/− platelets, whereas CLEC-2 levels were slightly elevated in Slap2−/− platelets (supplemental Table 1 and supplemental Figure 1A). The effect of SLAP or SLAP2 deficiency on platelet activation was analyzed by aggregation studies and flow cytometry measurements of agonist-induced inside-out activation of αIIbβ3 integrin and degranulation-dependent P-selectin surface exposure. Stimulation of platelets with GPVI-specific agonists (collagen-related peptide [CRP], the snake venom protein convulxin), or the CLEC-2 agonist rhodocytin, resulted in slightly elevated integrin activation and α-granule release, in particular at high agonist concentrations (supplemental Figure 1B-E). In contrast, activation responses to low concentrations of (hem)ITAM-specific agonists or to soluble agonists operating via G protein-coupled receptors, such as adenosine 5′-diphosphate (ADP), the thromboxane A2 analog U-46619, and thrombin, were normal. These results suggested a potential negative regulatory function for SLAP family proteins downstream of (hem)ITAM receptors in platelets, but this could be largely masked by functional compensation in the single knockouts.
Increased integrin activation, α− and dense-granule release, and aggregation in Slap−/−/Slap2−/− platelets upon GPVI or CLEC-2 stimulation. (A) Analysis of SLAP and SLAP2 expression in WT, Slap−/−, and Slap2−/− platelets by western blot. Actin expression was used as loading control and for quantification. Arrowheads indicate both SLAP2 isoforms (25 kDa and 28 kDa) in WT platelets. (B) Western blot analysis of GPVI and CLEC-2 expression in Slap−/−/Slap2−/− platelets. GPIIIa expression was used as loading control and for quantification. (A-B) Equal protein amounts were loaded and expression levels were quantified by densitometry with ImageJ software (National Institutes of Health) on 10 (A) or 12 (B) samples per genotype. Results represent mean ± SD. Expression levels were adjusted such that SLAP-to-actin, GPVI-to-GPIIIa, or CLEC-2-to-GPIIIa ratio in WT platelets was set to 1. (C) Flow cytometric analysis of activated integrin αIIbβ3 (binding of JON/A-PE) (left) and degranulation-dependent P-selectin exposure (right) upon stimulation with the indicated agonists in WT and Slap−/−/Slap2−/− platelets. Results are expressed as mean fluorescence intensity ± SD (n = 4 mice per group) and are representative of 6 independent experiments. Dividing lines indicate separate measurements. (D) Washed platelets were activated with the indicated agonists and light transmission was recorded on a Fibrintimer 4-channel aggregometer. ADP measurements were performed in platelet-rich plasma (PRP). Aggregation traces representative of 3 independent experiments with n = 4 are depicted. Arrows indicate addition of the respective agonist. (E) Washed platelets were incubated with Luciferase-Luciferin reagent and ATP release was measured in a Lumi-aggregometer upon stimulation with thrombin, CRP, or rhodocytin. Results are representative of 2 individual experiments with n = 4. Results represent mean ± SD. *P < .05; **P < .01; ***P < .001. A/U, ADP + U-46619; CRP, collagen-related peptide, CVX, convulxin; FITC, fluorescein isothiocyanate; PE, phycoerythrin; PRP, platelet-rich plasma; RC, rhodocytin; thr., thrombin; U46, U-46619.
Increased integrin activation, α− and dense-granule release, and aggregation in Slap−/−/Slap2−/− platelets upon GPVI or CLEC-2 stimulation. (A) Analysis of SLAP and SLAP2 expression in WT, Slap−/−, and Slap2−/− platelets by western blot. Actin expression was used as loading control and for quantification. Arrowheads indicate both SLAP2 isoforms (25 kDa and 28 kDa) in WT platelets. (B) Western blot analysis of GPVI and CLEC-2 expression in Slap−/−/Slap2−/− platelets. GPIIIa expression was used as loading control and for quantification. (A-B) Equal protein amounts were loaded and expression levels were quantified by densitometry with ImageJ software (National Institutes of Health) on 10 (A) or 12 (B) samples per genotype. Results represent mean ± SD. Expression levels were adjusted such that SLAP-to-actin, GPVI-to-GPIIIa, or CLEC-2-to-GPIIIa ratio in WT platelets was set to 1. (C) Flow cytometric analysis of activated integrin αIIbβ3 (binding of JON/A-PE) (left) and degranulation-dependent P-selectin exposure (right) upon stimulation with the indicated agonists in WT and Slap−/−/Slap2−/− platelets. Results are expressed as mean fluorescence intensity ± SD (n = 4 mice per group) and are representative of 6 independent experiments. Dividing lines indicate separate measurements. (D) Washed platelets were activated with the indicated agonists and light transmission was recorded on a Fibrintimer 4-channel aggregometer. ADP measurements were performed in platelet-rich plasma (PRP). Aggregation traces representative of 3 independent experiments with n = 4 are depicted. Arrows indicate addition of the respective agonist. (E) Washed platelets were incubated with Luciferase-Luciferin reagent and ATP release was measured in a Lumi-aggregometer upon stimulation with thrombin, CRP, or rhodocytin. Results are representative of 2 individual experiments with n = 4. Results represent mean ± SD. *P < .05; **P < .01; ***P < .001. A/U, ADP + U-46619; CRP, collagen-related peptide, CVX, convulxin; FITC, fluorescein isothiocyanate; PE, phycoerythrin; PRP, platelet-rich plasma; RC, rhodocytin; thr., thrombin; U46, U-46619.
Slap−/−/Slap2−/− platelets are hyperreactive to (hem)ITAM-specific agonists
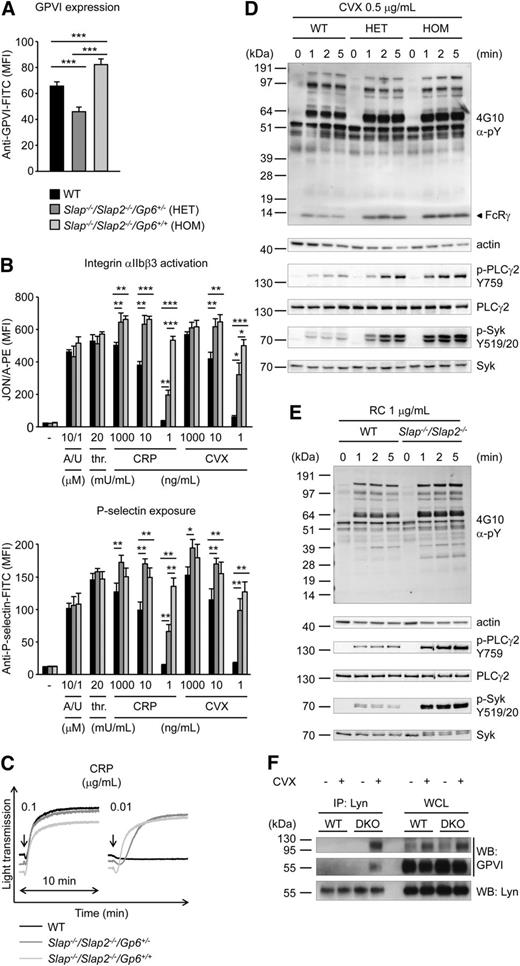
To assess a potential functional redundancy of the two adapter proteins, we generated SLAP/SLAP2 double-deficient mice. Slap−/−/Slap2−/− platelets exhibited a significant (P < .001) ∼23% increase in surface expression of GPVI and ∼15% increase in CLEC-2 compared with WT controls, as determined by flow cytometry (supplemental Table 2) and confirmed by western blot analyses (Figure 2B). Expression of all other major platelet surface receptors was unaltered (supplemental Table 2).
Slap−/−/Slap2−/− platelets displayed a marked hyperreactivity in response to GPVI or CLEC-2 stimulation (Figure 2C-E). This effect was best evident at subthreshold concentrations of GPVI agonists (CRP, convulxin and collagen) as well as the CLEC-2 agonist rhodocytin, which did not induce activation of WT platelets, but resulted in integrin activation, α- and dense-granule secretion, and robust aggregation of the mutant platelets. Importantly, activation responses to G protein-coupled receptor-specific agonists were unaltered in Slap−/−/Slap2−/− platelets (Figure 2C-E). Together, these results indicated that SLAP and SLAP2 have redundant functions in the regulation of (hem)ITAM signaling in platelets.
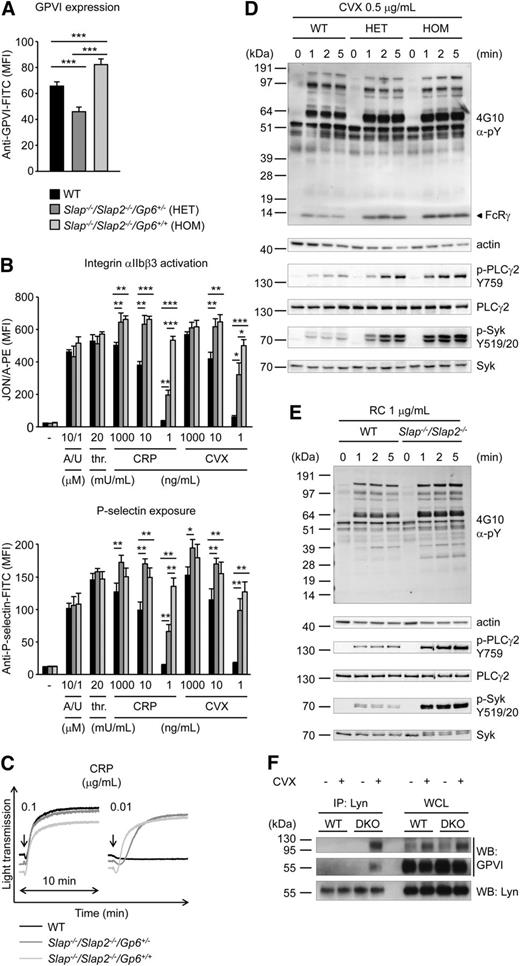
To examine whether increased GPVI/ITAM signaling in Slap−/−/Slap2−/− platelets can be explained by the elevated GPVI surface expression levels in SLAP/SLAP2 deficient mice, Slap−/−/Slap2−/−Gp6+/− were generated by crossing Slap−/−/Slap2−/− mice with Gp6−/− mice.13 Flow cytometric analysis confirmed that GPVI expression levels in Slap−/−/Slap2−/−/Gp6+/− platelets were reduced by approximately 50% compared with platelets of Slap−/−/Slap2−/−/Gp6+/+ litter-matched mice and were also significantly lower than in WT platelets (Figure 3A). Despite reduced GPVI surface expression levels, Slap−/−/Slap2−/−/Gp6+/− platelets were markedly hyperreactive to GPVI agonists, as shown by analysis of integrin αIIbβ3 activation, P-selectin exposure, and aggregation in response to GPVI-specific agonists, most notably at low and intermediate concentrations (Figure 3B-C). These results clearly demonstrated that the enhanced GPVI activation in SLAP/SLAP2 deficient platelets occurred independently of increased GPVI expression levels in these cells, and indicate a specific defect in the inhibition of GPVI signaling.
Increased GPVI/ITAM signaling in Slap−/−/Slap2−/− platelets occurs independently of elevated GPVI expression levels. (A) Analysis of GPVI expression levels in WT, Slap−/−/Slap2−/−/Gp6+/−, and Slap−/−/Slap2−/−/Gp6+/+ platelets. Diluted whole blood was stained with fluorescein isothiocyanate-labeled JAQ1 antibody and platelets were analyzed by flow cytometry (n = 4 mice per group). (B) Detection of integrin αIIbβ3 activation (binding of JON/A-PE) (top) and α-granule release (bottom) in response to the indicated agonists in WT, Slap−/−/Slap2−/−/Gp6+/−, and Slap−/−/Slap2−/−/Gp6+/+ platelets. Results are expressed as mean fluorescence intensity ± SD (n = 4 mice per group). (C) Washed platelets were activated with CRP and light transmission was monitored on a Fibrintimer 4-channel aggregometer. (D) WT, Slap−/−/Slap2−/−/Gp6+/− (HET), and Slap−/−/Slap2−/−/Gp6+/+ (HOM) platelets were stimulated with 0.5 μg/mL convulxin for the indicated time points. Whole-cell lysates were western-blotted and probed with the anti-phosphotyrosine antibody 4G10 or with phospho-specific antibodies. Staining of the respective nonphosphorylated proteins and actin served as loading control. (E) WT and Slap−/−/Slap2−/− platelets were stimulated with 1 μg/mL rhodocytin and analysis of tyrosine phosphorylation patterns was performed as described for (D). (F) SLAP/SLAP2 attenuate Lyn binding to activated GPVI. Washed WT and Slap−/−/Slap2−/− (DKO) platelets were left unstimulated or stimulated with 0.5 μg/mL convulxin for 20 seconds, lysed, and proteins immunoprecipitated (IP) with Lyn were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under nonreducing conditions and immunoblotted (WB) for GPVI or Lyn. An immunoblot of whole-cell lysate (WCL) loaded at 0.4% of Lyn immunoprecipitation input is also shown. Note that GPVI is detected as an ∼65 kDa monomer and an ∼120 kDa dimer, respectively. Results in all panels are representative of 3 independent experiments. *P < .05; **P < .01; ***P < .001. A/U, ADP + U-46619; CVX, convulxin; CRP, collagen-related peptide; DKO, double knockout; FITC, fluorescein isothiocyanate; HOM, Slap−/−Slap2−/−Gp6+/+ platelets; PE, phycoerythrin; RC, rhodocytin; thr., thrombin; WB, western blot; WCL, whole-cell lysate.
Increased GPVI/ITAM signaling in Slap−/−/Slap2−/− platelets occurs independently of elevated GPVI expression levels. (A) Analysis of GPVI expression levels in WT, Slap−/−/Slap2−/−/Gp6+/−, and Slap−/−/Slap2−/−/Gp6+/+ platelets. Diluted whole blood was stained with fluorescein isothiocyanate-labeled JAQ1 antibody and platelets were analyzed by flow cytometry (n = 4 mice per group). (B) Detection of integrin αIIbβ3 activation (binding of JON/A-PE) (top) and α-granule release (bottom) in response to the indicated agonists in WT, Slap−/−/Slap2−/−/Gp6+/−, and Slap−/−/Slap2−/−/Gp6+/+ platelets. Results are expressed as mean fluorescence intensity ± SD (n = 4 mice per group). (C) Washed platelets were activated with CRP and light transmission was monitored on a Fibrintimer 4-channel aggregometer. (D) WT, Slap−/−/Slap2−/−/Gp6+/− (HET), and Slap−/−/Slap2−/−/Gp6+/+ (HOM) platelets were stimulated with 0.5 μg/mL convulxin for the indicated time points. Whole-cell lysates were western-blotted and probed with the anti-phosphotyrosine antibody 4G10 or with phospho-specific antibodies. Staining of the respective nonphosphorylated proteins and actin served as loading control. (E) WT and Slap−/−/Slap2−/− platelets were stimulated with 1 μg/mL rhodocytin and analysis of tyrosine phosphorylation patterns was performed as described for (D). (F) SLAP/SLAP2 attenuate Lyn binding to activated GPVI. Washed WT and Slap−/−/Slap2−/− (DKO) platelets were left unstimulated or stimulated with 0.5 μg/mL convulxin for 20 seconds, lysed, and proteins immunoprecipitated (IP) with Lyn were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under nonreducing conditions and immunoblotted (WB) for GPVI or Lyn. An immunoblot of whole-cell lysate (WCL) loaded at 0.4% of Lyn immunoprecipitation input is also shown. Note that GPVI is detected as an ∼65 kDa monomer and an ∼120 kDa dimer, respectively. Results in all panels are representative of 3 independent experiments. *P < .05; **P < .01; ***P < .001. A/U, ADP + U-46619; CVX, convulxin; CRP, collagen-related peptide; DKO, double knockout; FITC, fluorescein isothiocyanate; HOM, Slap−/−Slap2−/−Gp6+/+ platelets; PE, phycoerythrin; RC, rhodocytin; thr., thrombin; WB, western blot; WCL, whole-cell lysate.
To study the function of SLAP/SLAP2 in ITAM signaling in more detail, we assessed changes in protein tyrosine phosphorylation in WT, Slap−/−/Slap2−/−/Gp6+/−, and Slap−/−/Slap2−/−/Gp6+/+ platelets upon stimulation with convulxin (Figure 3D). We detected enhanced and sustained tyrosine phosphorylation of key signaling molecules in the GPVI/ITAM activation pathway, including FcRγ-chain, Syk (Y519/520), and PLCγ2 (Y759) in SLAP/SLAP2 deficient platelets, which was also seen in Slap−/−/Slap2−/−/Gp6+/− platelets, and thus largely independent of GPVI expression levels (Figure 3D and supplemental Figure 2A). Notably, markedly enhanced tyrosine phosphorylation was observed also upon stimulation of SLAP/SLAP2 deficient platelets with rhodocytin (Figure 3E and supplemental Figure 2B).
To address the mechanism by which SLAP proteins inhibit GPVI signaling, we hypothesized that SLAP proteins might compete with SFKs for binding to the GPVI/FcRγ signaling complex. This is because Lyn and other SFKs are known to interact with GPVI,6,35 and because of the sequence similarity between Lyn and SLAP/SLAP2 (33% to 34% identity for SH3 and 51% identity for SH2 domains). Indeed, we detected strongly increased binding of Lyn to activated GPVI in the absence of SLAP/SLAP2 compared with control (Figure 3F), consistent with the increased tyrosine phosphorylation of downstream signaling proteins (Figure 3D).
Markedly enhanced GPVI-dependent procoagulant activity in Slap−/−/Slap2−/− platelets
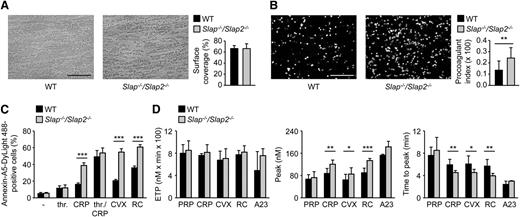
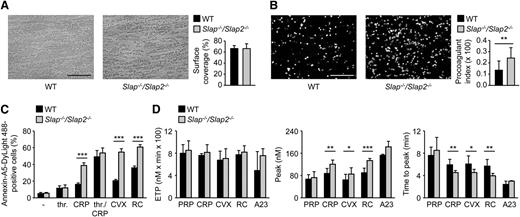
We next investigated consequences of SLAP/SLAP2 deficiency on platelet adhesion to collagen and aggregate formation under flow using a whole blood perfusion system. Unexpectedly, WT and Slap−/−/Slap2−/− platelets formed large aggregates to the same extent and with the same kinetics at intermediate shear rates of 1000 s−1 (supplemental Figure 3A-B).
GPVI signaling leads to a marked increase in cytosolic Ca2+ concentrations, subsequent surface exposure of procoagulant PS, and resultant thrombin generation.4,36 To test whether SLAP/SLAP2 regulate coagulant activity of platelets, blood from WT and Slap−/−/Slap2−/− mice was anticoagulated, perfused over immobilized collagen at a shear rate of 1000 s−1, and PS exposure was determined by Annexin-A5 staining. The surface covered by collagen-adherent platelets was similar in WT and mutant samples, whereas PS exposure was markedly increased in Slap−/−/Slap2−/− blood (Figure 4A-B). Enhanced GPVI-dependent procoagulant activity of Slap−/−/Slap2−/− platelets was confirmed by flow cytometric analysis of Annexin-A5-positive cells upon stimulation with different agonists (Figure 4C and supplemental Figure 3C). In agreement with enhanced PS-exposure, lack of SLAP/SLAP2 resulted in altered maximal amount of newly generated thrombin and significantly accelerated response upon stimulation with (hem)ITAM-specific agonists, whereas the overall amount of thrombin produced was comparable to the values in WT control samples (Figure 4D). Importantly, SLAP/SLAP2 deficiency did not affect plasma coagulation, as confirmed by unaltered activated plasma thromboplastin time and prothrombin time in Slap−/−/Slap2−/− mice compared with the WT control (supplemental Figure 4).
SLAP/SLAP2 deficiency enhances procoagulant activity and (hem)ITAM-dependent thrombin generation in platelets. (A) Unaltered surface coverage by collagen-adherent platelets in WT and Slap−/−/Slap2−/− blood. Representative phase-contrast images are depicted. (B) Increased PS exposure of Slap−/−/Slap2−/− platelets, as demonstrated by Annexin-A5-DyLight 488 staining of platelets. Procoagulant index indicates the ratio of surface coverage of PS-exposing platelets (Annexin-A5-DyLight 488 staining of platelets) to the total surface covered by platelets. Results in (A) and (B) are representative of 2 independent experiments with n ≥ 6. Images were obtained at ×40. Bars represent 50 μm. (C) Washed platelets were stimulated with 0.1 U/mL thrombin, 20 μg/mL CRP, a combination of both thrombin and CRP, 1 μg/mL convulxin, or 0.12 μg/mL rhodocytin, stained with saturating amounts of Annexin-A5-DyLight 488, and directly analyzed by flow cytometry. Results are representative of 2 independent experiments with n = 5. (D) Citrate-anticoagulated PRP was left unstimulated, or platelets were activated by incubation with convulxin (1 μg/mL), CRP (20 μg/mL), rhodocytin (1 μg/mL), or the Ca2+ ionophore A23187 (A23) (10 μM) for 10 minutes at 37°C. Thrombin generation was triggered with tissue factor/CaCl2. Endogenous thrombin potential (left); quantification of thrombin peak height (middle); and quantification of time to peak (right). n = 3 for A23187; n = 7 for CRP and RC; and n = 10 for PRP and CVX. *P < .05; **P < .01; ***P < .001. A23, A23187; CRP, collagen-related peptide; CVX, convulxin; ETP, endogenous thrombin potential; PRP, platelet-rich plasma; RC, rhodocytin; thr., thrombin.
SLAP/SLAP2 deficiency enhances procoagulant activity and (hem)ITAM-dependent thrombin generation in platelets. (A) Unaltered surface coverage by collagen-adherent platelets in WT and Slap−/−/Slap2−/− blood. Representative phase-contrast images are depicted. (B) Increased PS exposure of Slap−/−/Slap2−/− platelets, as demonstrated by Annexin-A5-DyLight 488 staining of platelets. Procoagulant index indicates the ratio of surface coverage of PS-exposing platelets (Annexin-A5-DyLight 488 staining of platelets) to the total surface covered by platelets. Results in (A) and (B) are representative of 2 independent experiments with n ≥ 6. Images were obtained at ×40. Bars represent 50 μm. (C) Washed platelets were stimulated with 0.1 U/mL thrombin, 20 μg/mL CRP, a combination of both thrombin and CRP, 1 μg/mL convulxin, or 0.12 μg/mL rhodocytin, stained with saturating amounts of Annexin-A5-DyLight 488, and directly analyzed by flow cytometry. Results are representative of 2 independent experiments with n = 5. (D) Citrate-anticoagulated PRP was left unstimulated, or platelets were activated by incubation with convulxin (1 μg/mL), CRP (20 μg/mL), rhodocytin (1 μg/mL), or the Ca2+ ionophore A23187 (A23) (10 μM) for 10 minutes at 37°C. Thrombin generation was triggered with tissue factor/CaCl2. Endogenous thrombin potential (left); quantification of thrombin peak height (middle); and quantification of time to peak (right). n = 3 for A23187; n = 7 for CRP and RC; and n = 10 for PRP and CVX. *P < .05; **P < .01; ***P < .001. A23, A23187; CRP, collagen-related peptide; CVX, convulxin; ETP, endogenous thrombin potential; PRP, platelet-rich plasma; RC, rhodocytin; thr., thrombin.
SLAP/SLAP2 limit pathological thrombus formation
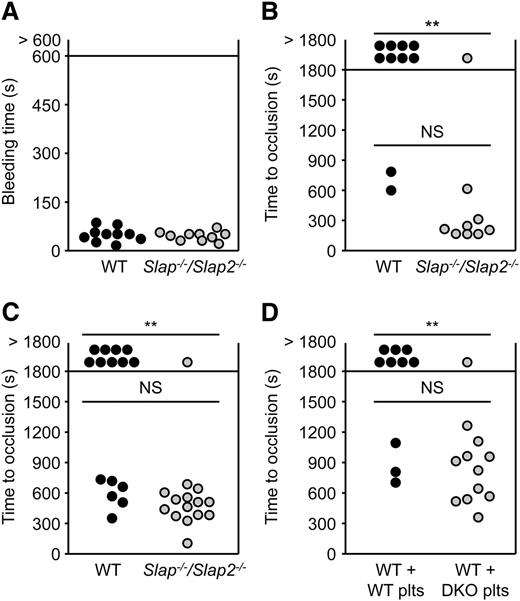
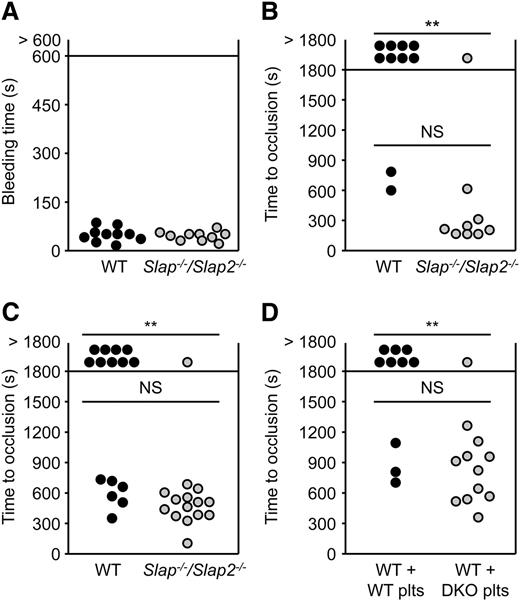
We next studied the role of SLAP and SLAP2 in hemostasis and thrombosis. Combined deficiency of SLAP and SLAP2 did not affect platelet hemostatic functions (mean tail bleeding time: 54 ± 22 seconds in WT mice, and 49 ± 14 seconds in Slap−/−/Slap2−/− mice; P = .6) (Figure 5A).
SLAP/SLAP2 limit pathological thrombus formation. (A) Unaltered hemostasis in Slap−/−/Slap2−/− mice. Tail bleeding times of WT and Slap−/−/Slap2−/− mice in saline at 37°C. Each symbol represents 1 animal. (B) The abdominal aorta was mechanically injured using a forceps (compression for 10 seconds) and blood flow was monitored by an ultrasonic flow probe until complete vessel occlusion for at least 5 minutes, or for a maximum of 30 minutes. Each symbol represents 1 animal. (C) The right carotid artery of WT and Slap−/−/Slap2−/− mice was injured by topical application of 2.5% FeCl3 for 90 seconds and blood flow was monitored with a Doppler flow probe until complete vessel occlusion for at least 2 minutes, or for a maximum of 30 minutes. Each symbol represents 1 animal. (D) WT mice were treated with a platelet-depleting anti-GPIbα antibody. Platelet-depleted WT mice were transfused with WT or Slap−/−/Slap2−/− (DKO) platelets and 7.5% FeCl3 were topically applied to the right carotid arteries of these mice for 1 minute. Time to complete vessel occlusion was determined as described for (C). Each symbol represents 1 animal. **P < .01. DKO, double knockout; NS, not significant.
SLAP/SLAP2 limit pathological thrombus formation. (A) Unaltered hemostasis in Slap−/−/Slap2−/− mice. Tail bleeding times of WT and Slap−/−/Slap2−/− mice in saline at 37°C. Each symbol represents 1 animal. (B) The abdominal aorta was mechanically injured using a forceps (compression for 10 seconds) and blood flow was monitored by an ultrasonic flow probe until complete vessel occlusion for at least 5 minutes, or for a maximum of 30 minutes. Each symbol represents 1 animal. (C) The right carotid artery of WT and Slap−/−/Slap2−/− mice was injured by topical application of 2.5% FeCl3 for 90 seconds and blood flow was monitored with a Doppler flow probe until complete vessel occlusion for at least 2 minutes, or for a maximum of 30 minutes. Each symbol represents 1 animal. (D) WT mice were treated with a platelet-depleting anti-GPIbα antibody. Platelet-depleted WT mice were transfused with WT or Slap−/−/Slap2−/− (DKO) platelets and 7.5% FeCl3 were topically applied to the right carotid arteries of these mice for 1 minute. Time to complete vessel occlusion was determined as described for (C). Each symbol represents 1 animal. **P < .01. DKO, double knockout; NS, not significant.
To assess the extent to which the above-described defects affect thrombotic events in vivo, Slap−/−/Slap2−/− and WT mice were subjected to two models of occlusive arterial thrombus. In the first model, the abdominal aorta is mechanically injured and thrombus formation occurs mainly through collagen-dependent mechanisms. The severity of injury was adjusted to achieve stable vessel occlusion in only 20% of the WT mice. In marked contrast, the majority (89%) of Slap−/−/Slap2−/− mice formed stable vessel occlusion, which also occurred considerably faster (282 ± 151 seconds in Slap−/−/Slap2−/− mice vs 713 ± 131 seconds in WT mice) (Figure 5B). In a second model, the carotid artery was injured by topical application of a low concentration of FeCl3. Stable vessel occlusion occurred in 93% of all tested Slap−/−/Slap2−/− mice, whereas 60% of the WT mice (9 out of 15) were not able to form occlusive thrombi within the observation period of 30 minutes (P < .01); mean time to occlusion: 607 ± 146 seconds in WT mice vs 483 ± 149 seconds in Slap−/−/Slap2−/− mice (Figure 5C). Importantly, similar results were obtained upon adoptive platelet transfer of Slap−/−/Slap2−/− platelets into platelet-depleted WT mice, thus excluding a contribution of other cell types than platelets to the observed phenotype (stable vessel occlusion in 30% of mice transfused with WT platelets, vessel occlusion in 92% of mice transfused with Slap−/−/Slap2−/− platelets; P < .01) (Figure 5D). Notably, application of a very low concentration of FeCl3 resulted in the formation of occlusive platelet-rich thrombi in Slap−/−/Slap2−/− mice, which did not differ morphologically from those observed in WT control mice treated with a higher concentration of FeCl3, demonstrating that thrombus formation in Slap−/−/Slap2−/− mice follows the same mechanisms as in WT mice, but occurs at a markedly lower threshold stimulus (supplemental Figure 5). Together, these findings demonstrated that SLAP/SLAP2 deficiency in platelets resulted in a prothrombotic phenotype, confirming the notion that these adapter proteins limit platelet activation in vivo, and thereby efficiently prevent occlusive thrombus formation and infarction.
SLAP/SLAP2 deficiency in platelets dramatically aggravates neurologic damage after focal cerebral ischemia
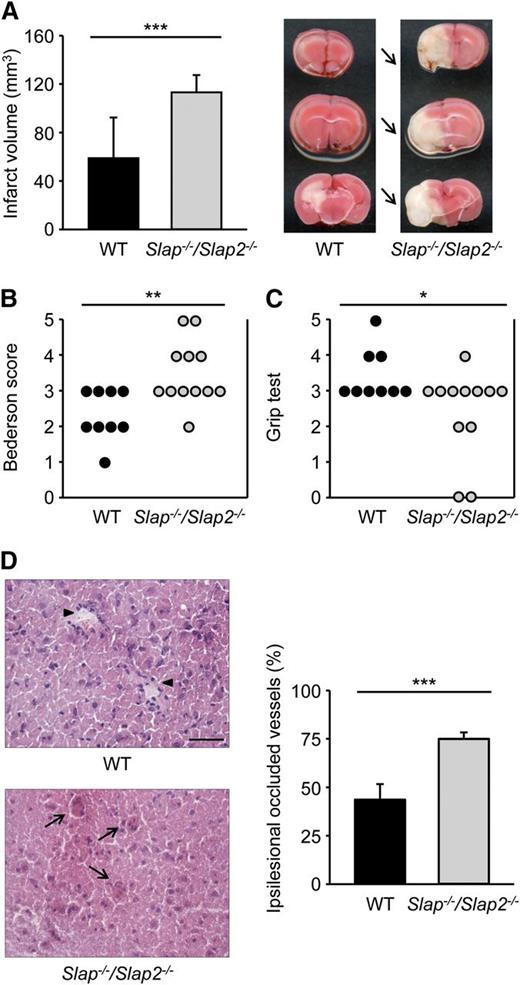
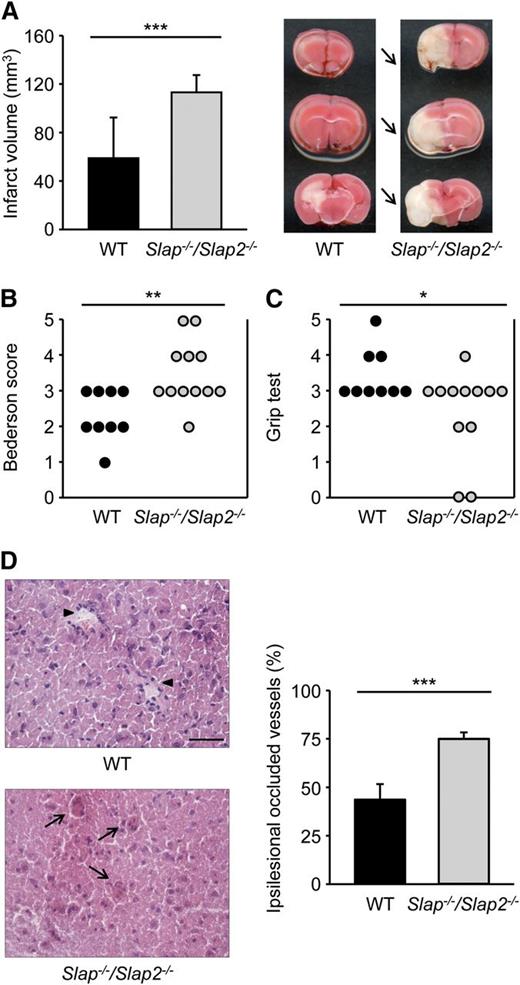
Ischemic stroke is a complex disease with multiple cellular interactions involved in the progression of thromboembolic vessel occlusion to infarct development.2 Although the exact mechanisms by which platelets contribute to the development of ischemic brain infarction are still poorly understood, there is increasing evidence that GPVI (and GPIb) plays a major role in this process.29,37 To determine the effect of SLAP/SLAP2 deficiency on cerebral infarct development, Slap−/−/Slap2−/− and WT mice were subjected to the tMCAO model of acute stroke where the MCA is occluded by a filament for 30 minutes, thereby reducing cerebral flow by >90%.29 In agreement with previous reports,38 WT mice developed only small infarctions after this short ischemia. In sharp contrast, infarct volumes in Slap−/−/Slap2−/− mice were increased by ∼92% compared with WT mice (58.8 ± 33.5 mm3 in WT mice vs 113.1 ± 14.3 mm3 in Slap−/−/Slap2−/− mice; P < .001) (Figure 6A). The increase in infarct size was functionally relevant, as it was associated with an overall impairment of neurologic and motor function, demonstrated by the significantly worsened outcome in the Bederson score and grip test, respectively (Figure 6B-C). The dramatic increase in infarct volume was accompanied by enhanced intracerebral thrombosis, as demonstrated by the markedly elevated number of occlusive vessels (Figure 6D). In agreement with the higher proportion of thrombotic vessels, immunohistochemical and western blot analyses revealed increased accumulation of fibrin(ogen) in the infarcted basal ganglia and cortices of Slap−/−/Slap2−/− mice (data not shown).
SLAP/SLAP2 deficiency dramatically aggravates neurologic damage after focal cerebral ischemia. Infarct volumes (A) and functional outcome (B-C) 24 hours after focal cerebral ischemia in WT and Slap−/−/Slap2−/− mice were investigated in a murine model of ischemic stroke. Mice were subjected to 30 minutes of tMCAO. (A) Brain infarct volumes in WT (n = 9) and Slap−/−/Slap2−/− (n = 12) mice were measured by planimetry (left). Results represent mean ± SD. Representative images of 3 coronal brain sections stained with 2,3,5-triphenyltetrazolium chloride for 24 hours after 30 minutes of tMCAO (right). Arrows indicate infarcted areas in Slap−/−/Slap2−/− mice. Bederson score (B) and grip test (C) were determined 24 hours after tMCAO. Each symbol represents 1 animal. (D) SLAP/SLAP2 deficiency dramatically increases microvascular thrombosis after 30 minutes of tMCAO. Representative hematoxylin and eosin stains from the ipsilesional hemispheres of WT (n = 6) and Slap−/−/Slap2−/− (n = 8) mice (left), and determination of the percentage of occluded vessels (right). Number of thrombotic vessels was significantly increased in Slap−/−/Slap2−/− mice (arrows), whereas the microvascular patency was largely preserved in WT mice (arrowheads). Images were obtained at ×40. Bar represents 50 μm. *P < .05; **P < .01; ***P < .001.
SLAP/SLAP2 deficiency dramatically aggravates neurologic damage after focal cerebral ischemia. Infarct volumes (A) and functional outcome (B-C) 24 hours after focal cerebral ischemia in WT and Slap−/−/Slap2−/− mice were investigated in a murine model of ischemic stroke. Mice were subjected to 30 minutes of tMCAO. (A) Brain infarct volumes in WT (n = 9) and Slap−/−/Slap2−/− (n = 12) mice were measured by planimetry (left). Results represent mean ± SD. Representative images of 3 coronal brain sections stained with 2,3,5-triphenyltetrazolium chloride for 24 hours after 30 minutes of tMCAO (right). Arrows indicate infarcted areas in Slap−/−/Slap2−/− mice. Bederson score (B) and grip test (C) were determined 24 hours after tMCAO. Each symbol represents 1 animal. (D) SLAP/SLAP2 deficiency dramatically increases microvascular thrombosis after 30 minutes of tMCAO. Representative hematoxylin and eosin stains from the ipsilesional hemispheres of WT (n = 6) and Slap−/−/Slap2−/− (n = 8) mice (left), and determination of the percentage of occluded vessels (right). Number of thrombotic vessels was significantly increased in Slap−/−/Slap2−/− mice (arrows), whereas the microvascular patency was largely preserved in WT mice (arrowheads). Images were obtained at ×40. Bar represents 50 μm. *P < .05; **P < .01; ***P < .001.
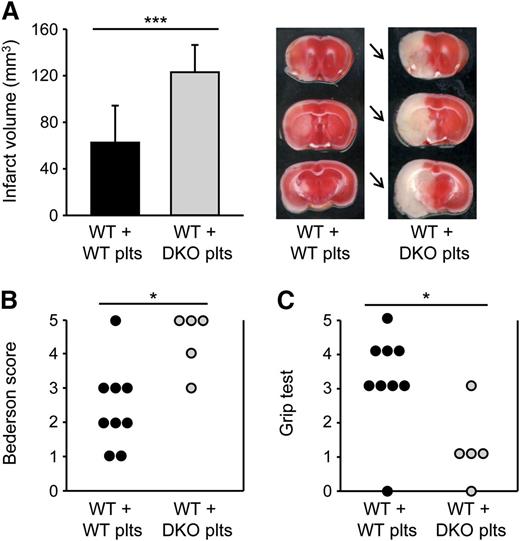
To specifically investigate the role of platelet SLAP and SLAP2 in the progression of ischemic stroke, we performed adoptive platelet transfer into platelet-depleted WT mice. Importantly, the transfused platelet populations were free of leukocytes, as tested by flow cytometry, microscopical inspection, and automated blood cell analysis. Furthermore, flow cytometric and transmission electron microscopy analyses confirmed that the platelet suspensions were also free of (leukocyte- or platelet-derived) microparticles. Strikingly, infarct volumes in WT mice transfused with Slap−/−/Slap2−/− platelets were dramatically increased by ∼97% compared with WT mice transfused with WT platelets (123.0 ± 23.4 mm3 vs 62.6 ± 31.8 mm3; P < .001) (Figure 7A), and this was accompanied by severe neurologic deficits (Figure 7B-C). These findings demonstrated that SLAP/SLAP2 activity in platelets is critically involved in limiting infarct growth following focal cerebral ischemia.
SLAP/SLAP2 deficiency in platelets induces a dramatic increase in infarct volume in a model of ischemic stroke. Infarct volumes (A) and functional outcome (B-C) 24 hours after focal cerebral ischemia in WT mice reconstituted with WT or Slap−/−/Slap2−/− (DKO) platelets after depletion of endogenous platelets were investigated in a murine model of ischemic stroke. Mice were subjected to 30 minutes of tMCAO. (A) Brain infarct volumes in platelet-depleted WT mice transfused with WT (n = 12) or Slap−/−/Slap2−/− (n = 8) platelets were measured by planimetry (left). Results represent mean ± SD. Representative images of 3 coronal brain sections stained with 2,3,5-triphenyltetrazolium chloride for 24 hours after 30 minutes of tMCAO (right). Arrows indicate infarcted areas in WT mice recipients of Slap−/−/Slap2−/− platelets. Bederson score (B) and grip test (C) were determined 24 hours after tMCAO. Each symbol represents 1 animal. *P < .05; ***P < .001. DKO, double knockout; plts, platelets.
SLAP/SLAP2 deficiency in platelets induces a dramatic increase in infarct volume in a model of ischemic stroke. Infarct volumes (A) and functional outcome (B-C) 24 hours after focal cerebral ischemia in WT mice reconstituted with WT or Slap−/−/Slap2−/− (DKO) platelets after depletion of endogenous platelets were investigated in a murine model of ischemic stroke. Mice were subjected to 30 minutes of tMCAO. (A) Brain infarct volumes in platelet-depleted WT mice transfused with WT (n = 12) or Slap−/−/Slap2−/− (n = 8) platelets were measured by planimetry (left). Results represent mean ± SD. Representative images of 3 coronal brain sections stained with 2,3,5-triphenyltetrazolium chloride for 24 hours after 30 minutes of tMCAO (right). Arrows indicate infarcted areas in WT mice recipients of Slap−/−/Slap2−/− platelets. Bederson score (B) and grip test (C) were determined 24 hours after tMCAO. Each symbol represents 1 animal. *P < .05; ***P < .001. DKO, double knockout; plts, platelets.
In a control experiment, WT mice received leukocytes isolated from blood of Slap−/−/Slap2−/− or WT mice, and were then subjected to 30 minutes of tMCAO. Both groups of mice developed only small infarcts, clearly demonstrating that white blood cells are not responsible for the increased susceptibility of Slap−/−/Slap2−/− mice toward ischemic stroke (supplemental Figure 6). Furthermore, induction of local cytokine production, infiltration by T cells (which have been shown to contribute to infarct growth in this model),39,40 complement activation destabilization of the blood-brain barrier were comparable in WT and Slap−/−/Slap2−/− mice for 24 hours after 30 minutes of tMCAO (data not shown). Together, these results further corroborated the notion that enhanced thrombotic, rather than proinflammatory activity, is a major determinant of the increased susceptibility of the mutant mice toward ischemic brain infarction.
Discussion
In this study, we have shown that SLAP and SLAP2 negatively regulate surface expression levels and signaling of the ITAM receptor GPVI and the hemITAM receptor CLEC-2 in platelets. However, single deficiency of either adapter protein had only a minor effect on platelet (hem)ITAM signaling, whereas the combined loss of SLAP and SLAP2 resulted in a marked platelet hyperreactivity to (hem)ITAM-coupled agonists in vitro, which translated into a significant prothrombotic phenotype and dramatically increased susceptibility to focal cerebral ischemia in vivo. Our results clearly demonstrate a functional redundancy of the two adapter proteins in the regulation of platelet (hem)ITAM signaling and reveal a crucial function of SLAP and SLAP2 in the control of thrombotic and thrombo-inflammatory events.
Involvement of SLAP in the regulation of surface expression levels of ITAM-coupled immune cell receptors, such as the TCR and BCR, is well-established.14 In vivo studies have demonstrated an important role for SLAP in regulating TCR expression during a specific T lymphocyte developmental stage at which repertoire selection takes place.20 Similarly, SLAP is required for the fine tuning of BCR levels and signal strength during B-cell development.18 Importantly, our data clearly demonstrate that increased GPVI receptor density was not responsible for the marked hyperreactivity, as this was also seen in SLAP/SLAP2 deficient Gp6+/− platelets, which expressed lower GPVI surface levels than WT control platelets (Figure 3A-D). This was supported by observations in a model cell line, in which expression of either SLAP or SLAP2 almost completely inhibited GPVI signaling without affecting expression levels (Figure 1A-B). We propose that the mechanism by which GPVI signaling is inhibited by SLAP proteins involves, at least in part, competition with SFKs for binding to the GPVI/FcRγ signaling complex. In support of this model, immunoprecipitation of the SFK Lyn contained substantially more GPVI in the absence of SLAP/SLAP2 (Figure 3F), and tyrosine phosphorylation of downstream signaling proteins was also elevated (Figure 3D). Collectively, these results establish SLAP/SLAP2 as negative regulators of ITAM signaling in platelets, presumably through competition with Lyn for binding to the GPVI cytosolic tail, and a similar mechanism may also apply to CLEC-2 signaling.
Our data further demonstrated that SLAP/SLAP2 significantly contributed to dampening collagen-induced procoagulant activity in platelets and ensuing thrombin generation (Figure 4). These results are in line with earlier studies indicating that key components of the GPVI/ITAM signaling cascade, including the FcRγ-chain, LAT, Syk, and PLCγ2, as well as GPVI-induced increase in cytosolic Ca2+ concentration, are critically involved in collagen-dependent PS exposure.28,36,41 Increasing evidence suggests that platelet procoagulant activity, in particular by propagation of the contact phase of coagulation, plays an important role in initiating arterial thrombus formation.1 Our results revealed that SLAP and SLAP2 are essential to prevent overshooting thrombotic and thrombo-inflammatory processes in vivo (Figures 5B-D, 6, and 7). Remarkably, using adoptive platelet transfer, we were able to unambiguously attribute this function to SLAP/SLAP2 expression in platelets and the contribution of both adapter proteins to attenuation of (hem)ITAM signaling.
Compelling evidence suggests that platelets are essentially involved in the progression of ischemic stroke, presumably by orchestrating a complex thrombo-inflammatory process, in which interactions between platelets, immune cells, and the contact activation system result in the disruption of the blood-brain barrier and neurologic damage.37,38 Although the underlying mechanisms of platelet contribution to infarct development are not completely understood, integrin αIIbβ3-dependent platelet aggregation was shown to be dispensable.29 By contrast, the importance of initial platelet adhesion and activation steps involving GPIb-von Willebrand factor interactions is now well-established.29,37,42-44 Likewise, GPVI also appears to play a crucial role in the pathophysiology of acute stroke, as shown by profound protection of GPVI-depleted mice in the tMCAO model of ischemic stroke,29 and indirect evidence indicating increased GPVI activity in acute stroke patients.45,46 Our findings further corroborate the hypothesis that GPVI/ITAM-mediated activation processes are of central importance during stroke progression. Significantly, GPVI has emerged as an attractive potential target for antithrombotic therapy, as its blockade or antibody-induced depletion provided powerful protection from experimental arterial thrombosis without affecting platelet hemostatic functions.4,5,47 Together, these experimental and clinical data emphasize a central role of GPVI in the course of acute ischemic disease states, and thereby establish modulators of GPVI activity, such as SLAP/SLAP2, as central factors in the pathology of atherothrombosis and ischemic stroke.
Taken together, our studies establish two distinct roles for the adapter proteins SLAP and SLAP2 in platelets. Firstly, SLAP/SLAP2 negatively regulate expression levels of two major platelet-activating receptors: the ITAM-containing collagen receptor GPVI/FcRγ-chain, which parallels SLAP/SLAP2 regulation of BCR and TCR levels, and the hemITAM-containing CLEC-2 receptor. Secondly, SLAP/SLAP2 are central regulators of (hem)ITAM signaling in platelets that are essential to limit arterial thrombus growth and thrombo-inflammatory processes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sarah Schieβl for help with thrombin generation assays, and Jonas Müller, Andrea Sauer, and Daniela Urlaub for excellent technical assistance.
This study was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 688) (B.N., G.S., and C.K.), the Rudolf Virchow Center, the Wellcome Trust (088410), a British Heart Foundation Senior Research Fellowship (FS/08/062/25797) (M.G.T.), and a British Society for Haemostasis and Thrombosis travel grant (M.G.T.). S.P.W. holds a British Heart Foundation chair position.
Authorship
Contribution: D.C. performed experiments, analyzed data, and wrote the manuscript; M.B., M.M., P.K., M.K.S., S.M.A., C.S.S., and C.E.H. performed experiments and analyzed data; C.K., G.S., L.L.D., and S.P.W. assisted with experimental design and contributed to the writing of the manuscript; and M.G.T. and B.N. designed and supervised research, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernhard Nieswandt, University Hospital Würzburg and Rudolf Virchow Center for Experimental Biomedicine, University of Würzburg; Josef-Schneider-Strasse 2, 97080 Würzburg, Germany; e-mail: bernhard.nieswandt@virchow.uni-wuerzburg.de; and Michael G. Tomlinson, School of Biosciences, College of Life and Environmental Sciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, United Kingdom; e-mail: m.g.tomlinson@bham.ac.uk.