Key Points
ECs express Tlr4 and Myd88 and, after in vivo LPS or E coli stimulation, are the prime sources of G-CSF.
ECs are sensors of systemically spread pathogens and subsequent drivers of BM emergency granulopoiesis.
Abstract
Systemic bacterial infection induces a hematopoietic response program termed “emergency granulopoiesis” that is characterized by increased de novo bone marrow (BM) neutrophil production. How loss of local immune control and bacterial dissemination is sensed and subsequently translated into the switch from steady-state to emergency granulopoiesis is, however, unknown. Using tissue-specific myeloid differentiation primary response gene 88 (Myd88)-deficient mice and in vivo lipopolysaccharide (LPS) administration to model severe bacterial infection, we here show that endothelial cells (ECs) but not hematopoietic cells, hepatocytes, pericytes, or BM stromal cells, are essential cells for this process. Indeed, ECs from multiple tissues including BM express high levels of Tlr4 and Myd88 and are the primary source of granulocyte colony-stimulating factor (G-CSF), the key granulopoietic cytokine, after LPS challenge or infection with Escherichia coli. EC-intrinsic MYD88 signaling and subsequent G-CSF production by ECs is required for myeloid progenitor lineage skewing toward granulocyte-macrophage progenitors, increased colony-forming unit granulocyte activity in BM, and accelerated BM neutrophil generation after LPS stimulation. Thus, ECs catalyze the detection of systemic infection into demand-adapted granulopoiesis.
Introduction
Granulocytes are generated from upstream bone marrow (BM) precursors under the control of various myeloid cytokines, most importantly granulocyte colony-stimulating factor (G-CSF).1 Among granulocytes, neutrophils are the dominant cell type in BM, peripheral blood (PB), and tissues in the steady state and during bacterial infection. Neutrophils serve as key first line of defense cells of the innate immune system.2 To exert their critical function, neutrophils are recruited to respective sites of infection.2 Although some neutrophils are able to reenter the vasculature,3-5 the majority of cells are consumed and undergo cell death in the inflamed tissue as a consequence of launching their antimicrobial defense mechanisms.2 In locally poorly controlled and consequently systemically spread microbial infection, neutrophils are in high demand and need to be regenerated in large numbers. Hence, because of their already short half-life in the steady state, ranging from a few hours to a few days,6 during severe systemic infection, steady-state granulopoiesis is switched to “emergency granulopoiesis” (ie, massively enhanced de novo neutrophil production in BM).7,8 Thus, besides efficient neutrophil recruitment to sites of infection and microbicidal neutrophil effector functions,2 the third critical component of a protective innate immune response is the ability of the hematopoietic system to launch the emergency granulopoiesis program.
A prerequisite for the initiation of emergency granulopoiesis is sensing of pathogen dissemination. Pattern-recognition receptors such as Toll-like receptors (TLRs) recognize respective conserved pathogen-associated molecular patterns as diverse as proteins, polysaccharides, and nucleic acids.9 TLRs can be found on the cell surface as well as within endosomal compartments, and TLR signal transduction occurs via adaptor molecules such as MYD88 and TRIF, leading to transcription of genes involved in the host response toward invading pathogens.10 TLR expression and function has been best characterized in mature immune effector cells.9 In addition, accumulating evidence has revealed that nonhematopoietic cells such as bladder epithelial cells,11 mesenchymal stromal cells,12,13 and endothelial cells (ECs)14,15 also express TLRs, thereby critically contributing to innate immune responses. Furthermore, it has been demonstrated that hematopoietic stem and progenitor cells (HSPCs) express some TLRs and directly respond to pathogen-associated molecular patterns with increased myelopoiesis and directed migration to inflamed sites.16-22 However, we have recently shown that direct pathogen sensing by HSPCs does not play an essential role in the immediate lipopolysaccharide (LPS)-induced emergency granulopoiesis response. By contrast, TLR4 agonist sensing by nonhematopoietic cells followed by granulopoietic growth factor release, primarily G-CSF, is the main route for the initiation of emergency granulopoiesis.23
We address here the fundamental question of which cell type out of the vast plethora of putative nonhematopoietic cells and tissues is the key sensor of systemically spread pathogens, specifically LPS and Escherichia coli, and how this sensing is translated into emergency granulopoiesis.
Methods
Mice
C57BL/6J (CD45.2+), B6.SJL-PtprcaPepcb/BoyJ (CD45.1+), Myd88−/−, Tlr4−/−, Trif−/−, LysM-Cre, Nes-Cre, Pdgfrb-Cre, Alb-Cre, Tie2-Cre, Myd88fll/fl (B6.129P2(SJL)-Myd88tm1Defr/J), and loxP-GFP (B6.Cg-Gt(ROSA)26Sortm6(CAGZsGreen1)Hze/J) mice were used in this study. All animals were maintained at the University Hospital Zurich animal facility and treated in accordance with guidelines of the Swiss Federal Veterinary Office. Experiments and procedures were approved by the Veterinäramt des Kantons, Zurich, Switzerland.
LPS injections, E coli infection, and G-CSF injections
Mice received 2 intraperitoneal injections with 35 μg ultrapure LPS from E coli 0111:B4 (InvivoGen) 48 hours apart and were analyzed 24 hours after the second injection. For some experiments, mice were administered 20 μg LPS 3 times (at 0, 12, and 20 hours) and were analyzed at time point 24 hours. Alternatively, mice were intraperitoneally (IP)-injected with 0.5 to 4.5 × 108E coli and analyzed 48 hours later. Some mice were IP-injected with 250 μg/kg body weight human G-CSF (Filgrastim; Amgen) 6 times in a 12-hour interval and analyzed as LPS-injected mice.
Flow cytometry
The following antibodies (all from eBiosciences, unless otherwise stated) were used to assess mature BM and PB cell populations: anti-CD11b (M1/70), anti-Gr1 (RB6-8C5), anti-Ly6-G (1A8; Biolegend), anti-Ly6-C (HK1.4), anti-B220 (RA3-6B2), and anti-CD3ε (145-2C11). For fluorescence-activated cell sorter (FACS) analysis of HSPCs, the following antibodies were used: anti-CD3ε (145-2C11), anti-CD4 (GK1.5), anti-CD8α (53-6.7), anti-B220 (RA3-6B2), anti-CD19 (MB19-1), anti-CD11b (M1/70), anti-Gr1 (RB6-8C5), anti-Ter119 (Ter-119), anti-IL7Rα (A7R34), anti-cKit (2B8), anti-Sca1 (D7), anti-CD34 (RAM34), anti-FcgR (93), and anti-NK1.1 (PK136; Becton Dickinson).
ECs were stained with anti-CD45 (30-F11), anti-TER119 (Ter-119), and anti-CD31 (390). In some experiments, anti-VE-cadherin (BV13), anti-Sca1 (E13-161.7; BD Biosciences), anti-VCAM1 (429), and anti-CD105 (MJ7/18) antibodies were used.
Classical and plasmacytoid dendritic cells were stained using anti-CD3ε (145-2C11), anti-CD19 (MB19-1), anti-NK1.1 (PK136; Becton Dickinson), anti-CD45RA (14.8; BD Biosciences), anti-CD11c (N418), and anti-MHCII (M5/114.15.2).
Hoechst33342 (Life Technologies) was used to exclude dead cells. Cells were analyzed or sorted on a FACS Canto II or FACS Aria III flow cytometer (BD Biosciences), respectively. Data were analyzed by FlowJo (Tree Star) software.
BrdU incorporation assay
Mice were injected with phosphate-buffered saline (PBS) or LPS according to the scheme depicted in Figure 5A. Twelve hours before analysis, mice received 1 single IP injection of 2 mg 5-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich). In addition, BrdU at a final concentration of 0.8 mg/mL as well as 5% glucose were added to the drinking water. Cells were isolated and further processed according to the manufacturer's protocol (BrdU Flow Kit; BD Pharmingen).
Colony-forming unit (CFU) assay
After in vivo PBS or LPS stimulation according to the scheme depicted in Figure 1A, 1 × 104 red blood cell–depleted whole BM cells were plated in methylcellulose (Methocult M3234; StemCell Technologies) mixed with Iscove's modified Dulbecco's medium (30% fetal calf serum, 2 mM l-glutamine, 50 μM 2-mercaptoethanol) with the following factors added: mIL3 (10 ng/mL), hIL6 (10 ng/mL), hIL11 (10 ng/mL), mFLT3-Ligand (10 ng/mL), mSCF (10 ng/mL), mGM-CSF (10 ng/mL), huTPO (50 ng/mL), and huEPO (4 U/mL) (all R&D Systems). Colonies were scored after 8 days of in vitro culture.
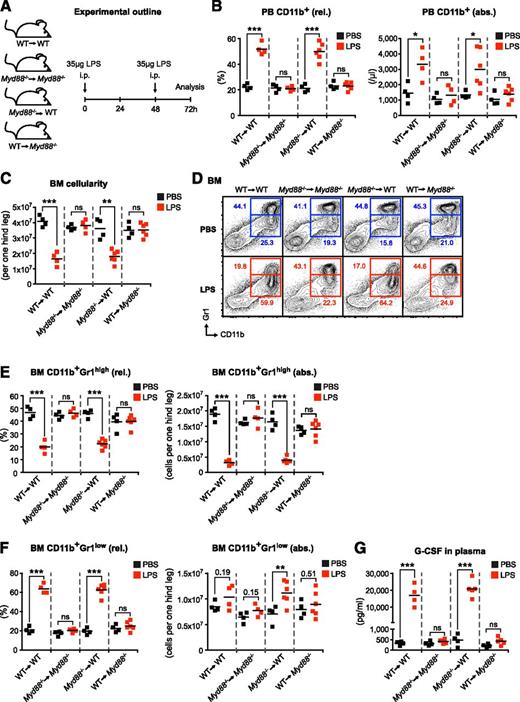
LPS-induced emergency granulopoiesis requires intact MYD88-mediated TLR signaling in nonhematopoietic cells. (A) Graphical scheme depicting experimental outline to induce LPS-induced emergency granulopoiesis in mice with MYD88 expression restricted to hematopoietic (WT→Myd88−/−) or nonhematopoietic (Myd88−/−→WT) cells, and respective control mice (WT→WT and Myd88−/−→Myd88−/−). (B) Frequency and absolute number of PB CD11b+ cells, and (C) BM cellularity after LPS stimulation in BM chimeric and control mice. (D) Representative FACS profile showing characteristic changes in BM CD11b+Gr1high mature and BM CD11b+Gr1low immature neutrophils after LPS stimulation. (E) Frequencies and absolute numbers of BM CD11b+Gr1high mature, (F) BM CD11b+Gr1low immature neutrophils, and (G) plasma G-CSF levels in reciprocal Myd88−/− BM chimeras upon systemic LPS injection. Black squares, PBS-injected mice; red squares, LPS-injected mice; rel., relative; abs., absolute. Data from 2 independent experiments are shown. Two-tailed Student t tests were used to assess statistical significance (*P < .05, **P < .01, ***P < .001; ns, nonsignificant).
LPS-induced emergency granulopoiesis requires intact MYD88-mediated TLR signaling in nonhematopoietic cells. (A) Graphical scheme depicting experimental outline to induce LPS-induced emergency granulopoiesis in mice with MYD88 expression restricted to hematopoietic (WT→Myd88−/−) or nonhematopoietic (Myd88−/−→WT) cells, and respective control mice (WT→WT and Myd88−/−→Myd88−/−). (B) Frequency and absolute number of PB CD11b+ cells, and (C) BM cellularity after LPS stimulation in BM chimeric and control mice. (D) Representative FACS profile showing characteristic changes in BM CD11b+Gr1high mature and BM CD11b+Gr1low immature neutrophils after LPS stimulation. (E) Frequencies and absolute numbers of BM CD11b+Gr1high mature, (F) BM CD11b+Gr1low immature neutrophils, and (G) plasma G-CSF levels in reciprocal Myd88−/− BM chimeras upon systemic LPS injection. Black squares, PBS-injected mice; red squares, LPS-injected mice; rel., relative; abs., absolute. Data from 2 independent experiments are shown. Two-tailed Student t tests were used to assess statistical significance (*P < .05, **P < .01, ***P < .001; ns, nonsignificant).
Quantitative reverse-transcription PCR
ECs were sorted directly into lysis buffer belonging to RNeasy Micro Kit (QIAGEN). Total RNA extraction was carried out according to the manufacturer’s protocol and subsequently subjected to reverse transcription using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantitative real-time polymerase chain reaction (PCR) was performed using SYBR green reagent (Applied Biosystems) and samples were run on a 7500 FAST real-time PCR thermal cycler (Applied Biosystems). Actb was used to normalize the RNA content of samples.
Enzyme-linked immunosorbent assay
PB was obtained from mice postmortem by cardiac puncture using heparinized syringes and centrifuged to obtain plasma. G-CSF plasma levels were measured using Quantikine ELISA kits according to the manufacturer's instructions (R&D Systems).
Equations and statistical analyses
Significance of differences was analyzed using Student t test. A difference between experimental groups was considered significant when the P value was <.05. All statistical analyses were calculated with Prism software (GraphPad Software, version 5.01).
Results
LPS-induced emergency granulopoiesis requires intact MYD88-mediated TLR signaling in nonhematopoietic cells
To examine whether MYD88 expression within hematopoietic or nonhematopoietic cells is required for LPS-induced emergency granulopoiesis, we generated BM chimeric mice with MYD88 expression restricted to either the hematopoietic (wild-type [WT]→Myd88−/−) or the nonhematopoietic cellular compartment (Myd88−/−→WT) as well as respective control mice (WT→WT and Myd88−/−→Myd88−/−) (Figure 1A). We IP-injected chimeric mice with high doses of LPS to mimic severe systemic infection (Figure 1A) and analyzed typical surrogate hallmarks of emergency granulopoiesis. Although absolute leukocyte counts did not change significantly in PB upon LPS stimulation (data not shown), the frequency and absolute numbers of PB CD11b+ cells significantly increased in WT→WT mice (Figure 1B). This response was completely absent in Myd88−/−→Myd88−/− mice, indicating that LPS-induced elevation in PB CD11b+ cells depends on MYD88 signaling. Strikingly, although WT→Myd88−/− mice were also completely nonresponsive toward LPS, Myd88−/−→WT mice showed a normal LPS response (Figure 1B). Next, we analyzed the BM of chimeric mice after LPS treatment. We found that total BM cell numbers are significantly decreased upon LPS stimulation in WT→WT mice (Figure 1C). The reduction of BM cellularity is mediated in a MYD88-dependent manner because Myd88−/−→Myd88−/− mice did not show this response. This depends on MYD88 expression within nonhematopoietic cells because Myd88−/−→WT mice responded normally but WT→Myd88−/− mice lacked this response (Figure 1C). A characteristic feature of emergency granulopoiesis is a significant, relative, and absolute decrease in BM CD11b+Gr1high mature neutrophils (Figure 1D-E). This response is paralleled by a significant relative increase in BM CD11b+Gr1low immature neutrophils23-25 and a trend toward an absolute increase in BM CD11b+Gr1low immature neutrophils. These reciprocal changes in BM CD11b+Gr1high mature neutrophils and BM CD11b+Gr1low immature neutrophils after LPS injection require intact MYD88 signaling in nonhematopoietic cells (Figure 1D-F).
Although various cytokines such as granulocyte-monocyte colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor, interleukin-6, and G-CSF have the capacity to stimulate both steady-state as well as emergency granulopoiesis, none of these cytokines is absolutely essential.1,26-29 However, G-CSF plays an outstanding role in these processes as demonstrated in studies of G-csf−/− and G-csfr−/− mice showing hematopoietic defects in steady state, and most importantly, upon infection.1,30,31 Notably, administration of recombinant G-CSF is routinely used to treat iatrogenic32 and congenital33 neutropenia. Furthermore, G-CSF alone is sufficient to induce a response indistinguishable from LPS-induced emergency granulopoiesis.23,34 We therefore assessed G-CSF plasma levels in BM chimeric mice after LPS stimulation. We found highly significant increases in plasma G-CSF levels in WT→WT and Myd88−/−→WT mice (Figure 1G). By contrast, Myd88−/−→Myd88−/− and WT→Myd88−/− mice did not show elevated plasma G-CSF levels. Thus, LPS-triggered G-CSF release requires intact Myd88 within nonhematopoietic cells.
To corroborate our findings on the importance of nonhematopoietic cells for the initiation of emergency granulopoiesis and to further rule out a role for tissue-resident macrophages for this process, we generated LysM-Cre;Myd88fl/fl mice with a Myd88 deficiency restricted to myeloid cells including macrophages.35 In line with the results on hematopoietic chimeric animals, we observed that LysM-Cre;Myd88fl/fl mice are fully capable to launch emergency granulopoiesis (supplemental Figure 1, available on the Blood Web site).
Altogether, our results on MYD88 and previous data on TLR423 unambiguously show that LPS-induced emergency granulopoiesis is initiated through TLR agonist sensing and MYD88-dependent signaling by nonhematopoietic cells.
LPS-induced emergency granulopoiesis is abrogated in Tie2-Cre;Myd88fl/fl mice
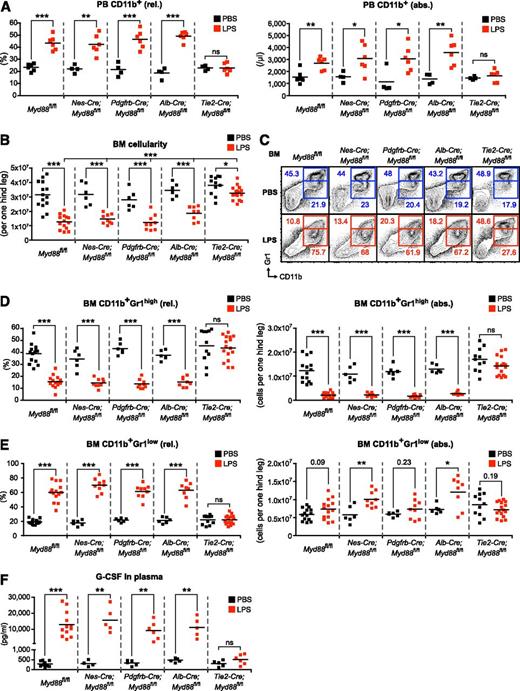
To unravel the identity of the nonhematopoietic cell type critical for emergency granulopoiesis, we used Cre-loxP recombination technology to delete Myd88 from various candidate nonhematopoietic cell populations to study their relative contribution to pathogen sensing (supplemental Table 1). To achieve Myd88 deletion in cells of the BM microenvironment (Nestin+ mesenchymal stem cells and perivascular stromal cells), we used Nes-Cre36-38 and Pdgfrb-Cre39 mice. Cells belonging to the vasculature (ECs and pericytes) were targeted using Tie2-Cre40 and Pdgfrb-Cre39 mice, respectively. We also employed Alb-Cre41 mice to target hepatocytes as a candidate parenchymal organ cell type. First, we assessed the LPS response in PB of control and tissue-specific Myd88−/− mice. We observed that the frequency and absolute numbers of PB CD11b+ cells significantly increased in LPS-injected Myd88fl/fl control, Nes-Cre;Myd88fl/fl, Pdgfrb-Cre;Myd88fl/fl, and Alb-Cre;Myd88fl/fl mice, whereas Tie2-Cre;Myd88fl/fl mice lacked the response (Figure 2A). The majority of PB CD11b+ cells could be identified as neutrophils based on coexpression of Ly6-G, whereas PB CD11b+Ly6-Chigh inflammatory monocytes showed a relative decrease but overall stable absolute numbers (supplemental Figure 2).
LPS-induced emergency granulopoiesis is abrogated in Tie2-Cre;Myd88fl/fl mice. (A) Frequency and absolute number of PB CD11b+ cells after LPS stimulation in control Myd88fl/fl, and experimental Nes-Cre;Myd88fl/fl, Pdgfrb-Cre;Myd88fl/fl, Alb-Cre;Myd88fl/fl, and Tie2-Cre;Myd88fl/fl mice. (B) BM cellularity after LPS stimulation in control Myd88fl/fl, and experimental Nes-Cre;Myd88fl/fl, Pdgfrb-Cre;Myd88fl/fl, Alb-Cre;Myd88fl/fl, and Tie2-Cre;Myd88fl/fl mice. (C) Representative FACS profile showing characteristic LPS-induced changes in BM CD11b+Gr1high mature and BM CD11b+Gr1low immature neutrophils in control Myd88fl/fl, and experimental Nes-Cre;Myd88fl/fl, Pdgfrb-Cre;Myd88fl/fl, Alb-Cre;Myd88fl/fl, 0and Tie2-Cre;Myd88fl/fl mice. (D) Frequencies and absolute numbers of BM CD11b+Gr1high mature and (E) BM CD11b+Gr1low immature neutrophils, and (F) plasma G-CSF levels in the different sets of tissue-specific Myd88−/− mice after systemic LPS injection. Black squares, PBS-injected mice; red squares, LPS-injected mice; rel., relative; abs., absolute. Data from at least 3 independent experiments are shown. Two-tailed Student t tests were used to assess statistical significance (*P < .05, **P < .01, ***P < .001).
LPS-induced emergency granulopoiesis is abrogated in Tie2-Cre;Myd88fl/fl mice. (A) Frequency and absolute number of PB CD11b+ cells after LPS stimulation in control Myd88fl/fl, and experimental Nes-Cre;Myd88fl/fl, Pdgfrb-Cre;Myd88fl/fl, Alb-Cre;Myd88fl/fl, and Tie2-Cre;Myd88fl/fl mice. (B) BM cellularity after LPS stimulation in control Myd88fl/fl, and experimental Nes-Cre;Myd88fl/fl, Pdgfrb-Cre;Myd88fl/fl, Alb-Cre;Myd88fl/fl, and Tie2-Cre;Myd88fl/fl mice. (C) Representative FACS profile showing characteristic LPS-induced changes in BM CD11b+Gr1high mature and BM CD11b+Gr1low immature neutrophils in control Myd88fl/fl, and experimental Nes-Cre;Myd88fl/fl, Pdgfrb-Cre;Myd88fl/fl, Alb-Cre;Myd88fl/fl, 0and Tie2-Cre;Myd88fl/fl mice. (D) Frequencies and absolute numbers of BM CD11b+Gr1high mature and (E) BM CD11b+Gr1low immature neutrophils, and (F) plasma G-CSF levels in the different sets of tissue-specific Myd88−/− mice after systemic LPS injection. Black squares, PBS-injected mice; red squares, LPS-injected mice; rel., relative; abs., absolute. Data from at least 3 independent experiments are shown. Two-tailed Student t tests were used to assess statistical significance (*P < .05, **P < .01, ***P < .001).
Next, we investigated the BM response toward LPS. Not only did we observe a decrease in BM cellularity in LPS-injected Myd88fl/fl control, Nes-Cre;Myd88fl/fl, Pdgfrb-Cre;Myd88fl/fl, and Alb-Cre;Myd88fl/fl mice (Figure 2B), we also revealed a reduction in the frequency and absolute number of BM CD11b+Gr1high mature neutrophils (Figure 2C-D). Although the percentage of BM CD11b+Gr1low immature neutrophils was highly significantly increased, there was only a minor increase in absolute numbers that, likely because of the higher variation in BM cellularity, did not always yield statistical significance (Figure 2C,E). Strikingly, Tie2-Cre;Myd88fl/fl mice were nonresponsive toward systemic LPS injection, with the exception of a small but significant decrease in BM cellularity that was, however, significantly reduced in magnitude compared with the other groups of mice (Figure 2B-E). In line with these findings, G-CSF levels increased massively, up to 100-fold, in control and respective tissue-specific Myd88−/− mice with the exception of Tie2-Cre;Myd88fl/fl mice that lacked a significant rise in plasma G-CSF concentration (Figure 2F).
ECs express high levels of Myd88 and Tlr4 and respond to LPS challenge with strong upregulation of VCAM-1 and G-csfin vivo
Our findings suggested that ECs, which are targeted in Tie2-Cre mice, are the cells within the nonhematopoietic compartment responsible for sensing LPS, translating this signal into G-CSF release, and consequently initiating emergency granulopoiesis.
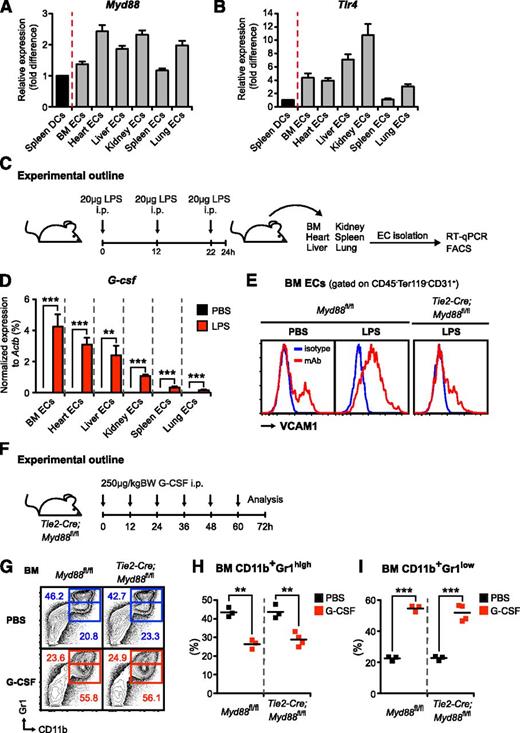
To assess expression of Tlr4 and Myd88 in ECs, we isolated CD45−Ter119−CD31+ ECs from BM, heart, lung, liver, spleen, and kidney and compared these with pooled spleen classical and plasmacytoid dendritic cells with defined TLR expression patterns.42 No immunophenotypical differences were observed between ECs from different organs with respect to the endothelial markers VE-cadherin, CD105, CD34, and Sca1 (data not shown). Myd88 and Tlr4 were abundantly expressed in ECs at similar or higher levels compared with dendritic cells, ie, cell populations known for their TLR expression profile (Figure 3A-B).20
Endothelial cells from various organs express high levels of Myd88 and Tlr4, respond to LPS challenge with strong upregulation of VCAM-1 and G-csf in vivo, and exogenous G-CSF administration rescues emergency granulopoiesis in Tie2-Cre;Myd88fl/fl mice. (A) Myd88 and (B) Tlr4 expression were assessed by quantitative reverse-transcription PCR in CD45−Ter119−CD31+ECs isolated from BM, heart, liver, kidney, spleen, and lung (gray bars) compared with spleen DCs (black bars), ie, pooled classical dendritic cells (CD3ε−CD19−NK1.1−CD11chighCD45RA−MHCII+) and plasmacytoid dendritic cells (CD3ε−CD19−NK1.1−CD11c+CD45RA+MHCIIhigh). All cells were isolated from steady-state mice. (C) Graphical scheme depicting experimental outline to assess in vivo LPS responsiveness of ECs that were flow-cytometrically sorted from BM, heart, liver, kidney, spleen, and lung of PBS- and LPS-injected mice, respectively. (D) Comparative G-csf transcript levels normalized to Actb in ECs from BM, heart, liver, kidney, spleen, and lung of LPS-injected (red bars) vs PBS-injected (black bars) wild-type mice. (E) Representative FACS analysis depicting cell-surface expression of VCAM1 (red lines) on BM ECs in steady state as well as LPS-injected control Myd88fl/fl and Tie2-Cre;Myd88fl/fl mice. Isotype control shown as blue line. (F) Graphical scheme showing experimental outline to assess G-CSF effects in vivo. (G) Representative FACS profile showing characteristic G-CSF–induced changes in BM CD11b+Gr1high mature and BM CD11b+Gr1low immature neutrophils in control Myd88fl/fl and Tie2-Cre;Myd88fl/fl mice. (H) Frequencies of BM CD11b+Gr1high mature and (I) BM CD11b+Gr1low immature neutrophils in PBS- and G-CSF–injected control Myd88fl/fl and Tie2-Cre;Myd88fl/fl mice. All data represent mean ± standard deviation from 2 or 3 independent experiments. Two-tailed Student t tests were used to assess statistical significance (**P < .01, ***P < .001).
Endothelial cells from various organs express high levels of Myd88 and Tlr4, respond to LPS challenge with strong upregulation of VCAM-1 and G-csf in vivo, and exogenous G-CSF administration rescues emergency granulopoiesis in Tie2-Cre;Myd88fl/fl mice. (A) Myd88 and (B) Tlr4 expression were assessed by quantitative reverse-transcription PCR in CD45−Ter119−CD31+ECs isolated from BM, heart, liver, kidney, spleen, and lung (gray bars) compared with spleen DCs (black bars), ie, pooled classical dendritic cells (CD3ε−CD19−NK1.1−CD11chighCD45RA−MHCII+) and plasmacytoid dendritic cells (CD3ε−CD19−NK1.1−CD11c+CD45RA+MHCIIhigh). All cells were isolated from steady-state mice. (C) Graphical scheme depicting experimental outline to assess in vivo LPS responsiveness of ECs that were flow-cytometrically sorted from BM, heart, liver, kidney, spleen, and lung of PBS- and LPS-injected mice, respectively. (D) Comparative G-csf transcript levels normalized to Actb in ECs from BM, heart, liver, kidney, spleen, and lung of LPS-injected (red bars) vs PBS-injected (black bars) wild-type mice. (E) Representative FACS analysis depicting cell-surface expression of VCAM1 (red lines) on BM ECs in steady state as well as LPS-injected control Myd88fl/fl and Tie2-Cre;Myd88fl/fl mice. Isotype control shown as blue line. (F) Graphical scheme showing experimental outline to assess G-CSF effects in vivo. (G) Representative FACS profile showing characteristic G-CSF–induced changes in BM CD11b+Gr1high mature and BM CD11b+Gr1low immature neutrophils in control Myd88fl/fl and Tie2-Cre;Myd88fl/fl mice. (H) Frequencies of BM CD11b+Gr1high mature and (I) BM CD11b+Gr1low immature neutrophils in PBS- and G-CSF–injected control Myd88fl/fl and Tie2-Cre;Myd88fl/fl mice. All data represent mean ± standard deviation from 2 or 3 independent experiments. Two-tailed Student t tests were used to assess statistical significance (**P < .01, ***P < .001).
To analyze the functional relevance of Tlr4 and Myd88 expression in ECs, we injected mice with LPS or PBS (Figure 3C); isolated ECs from BM, heart, lung, liver, spleen, and kidney; and assessed G-csf transcripts by quantitative real-time PCR. Although in the PBS-injected steady-state mice, G-csf was only detectable at very low levels, LPS injection resulted in a strong upregulation of G-csf (up to several hundred-fold) in ECs from all organs analyzed (Figure 3D). Interestingly, G-csf induction was relatively highest in ECs from BM (ie, the primary site of hematopoiesis). However, it needs to be emphasized that this does not necessarily imply that absolute G-CSF amounts produced are also highest in this tissue. Transcription and protein production do not always correlate, and quantity of protein produced in a given organ will also depend on frequency of producing cells at site.
To test whether there might be heterogeneity in the responsiveness toward LPS within different sections of the vasculature, we analyzed the activation status of ECs upon systemic LPS stimulation by measuring VCAM1 cell-surface expression.43 However, we did not detect differences between ECs isolated from different tissues and, importantly and in line with the observed nonresponsiveness, VCAM1 upregulation on ECs was abrogated in Tie2-Cre;Myd88fl/fl mice (Figure 3E and data not shown).
To confirm that disrupted MYD88 signaling in Tie2-Cre;Myd88fl/fl mice does not affect G-CSF signal transduction and consequently impairs emergency granulopoiesis, we injected Myd88fl/fl and Tie2-Cre;Myd88fl/fl mice with recombinant human G-CSF (Figure 3F). In accordance with our previously published data on G-CSF effects in Tlr4−/− mice,23 we observed that G-CSF alone without an additional inflammatory stimulus is sufficient to accurately mimic emergency granulopoiesis (Figure 3G-I and data not shown).
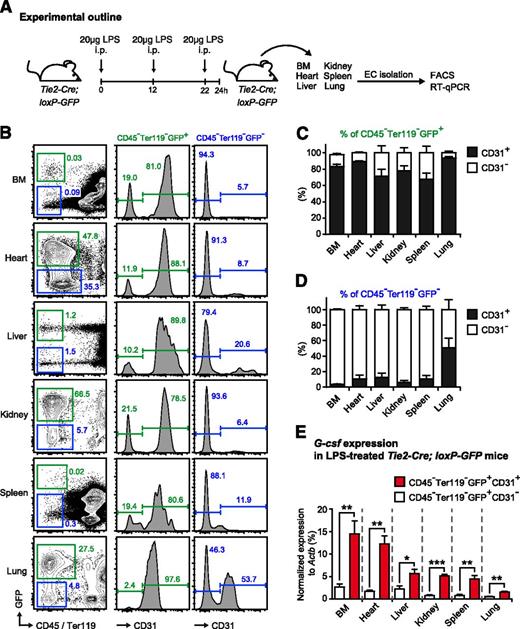
Given the possibility of insufficient tissue specificity of Cre-loxP technology, we generated Tie2-Cre-loxP-GFP reporter mice to study the effectiveness and specificity of EC targeting. To this end, we isolated nonhematopoietic cells from BM, heart, liver, kidney, spleen, and lung and analyzed both GFP and expression of the EC marker CD31 by FACS (Figure 4A-D). In accordance with previously published reports,37 we found that the vast majority of ECs (approximately 90%) were efficaciously targeted in Tie2-Cre-loxP-GFP reporter mice. This pattern was robustly observed in all tissues analyzed, except for the lung, where we found a higher fraction of CD31+ cells within the CD45−Ter119−GFP− fraction (Figure 4B-D). Furthermore, we observed that about 20% (with some variations between the different tissues) of CD45−Ter119−GFP+ cells in Tie2-Cre-loxP-GFP reporter mice do not express the EC marker CD31 and thus, presumably, are not ECs (Figure 4B-C).
Endothelial cells are effectively targeted in Tie2-Cre-loxP-GFP reporter mice and are the main source of G-csf after in vivo LPS stimulation. (A) Graphical scheme depicting experimental outline to induce LPS-induced emergency granulopoiesis and to assess G-csf expression in sorted CD45−Ter119−GFP+CD31+ ECs and CD45−Ter119−GFP+CD31− non-ECs isolated from different organs of Tie2-Cre-loxP-GFP reporter mice. (B) Representative FACS profile of GFP and CD31 expression within nonhematopoietic cells (CD45−Ter119−) isolated from BM, heart, liver, kidney, spleen, and lung of Tie2-Cre-loxP-GFP reporter mice. (C) Percentages of CD31+ (black bars) and CD31− (white bars) cells within the CD45−Ter119−GFP+ cell population in BM, heart, liver, kidney, spleen, and lung of Tie2-Cre-loxP-GFP reporter mice. (D) Percentages of CD31+ (black bars) and CD31− (white bars) cells within the CD45−Ter119−GFP− cell population in BM, heart, liver, kidney, spleen, and lung of Tie2-Cre-loxP-GFP reporter mice. (E) Comparative G-csf transcript levels normalized to Actb in sorted CD45−Ter119−GFP+CD31+ ECs (red bars) vs CD45−Ter119−GFP+CD31− non-ECs (white bars) isolated from BM, heart, liver, kidney, spleen, and lung of LPS-injected Tie2-Cre-loxP-GFP reporter mice. All data represent mean ± standard deviation from 2 independent experiments (n = 5 mice). Two-tailed Student t tests were used to assess statistical significance (*P < .05, **P < .01, ***P < .001).
Endothelial cells are effectively targeted in Tie2-Cre-loxP-GFP reporter mice and are the main source of G-csf after in vivo LPS stimulation. (A) Graphical scheme depicting experimental outline to induce LPS-induced emergency granulopoiesis and to assess G-csf expression in sorted CD45−Ter119−GFP+CD31+ ECs and CD45−Ter119−GFP+CD31− non-ECs isolated from different organs of Tie2-Cre-loxP-GFP reporter mice. (B) Representative FACS profile of GFP and CD31 expression within nonhematopoietic cells (CD45−Ter119−) isolated from BM, heart, liver, kidney, spleen, and lung of Tie2-Cre-loxP-GFP reporter mice. (C) Percentages of CD31+ (black bars) and CD31− (white bars) cells within the CD45−Ter119−GFP+ cell population in BM, heart, liver, kidney, spleen, and lung of Tie2-Cre-loxP-GFP reporter mice. (D) Percentages of CD31+ (black bars) and CD31− (white bars) cells within the CD45−Ter119−GFP− cell population in BM, heart, liver, kidney, spleen, and lung of Tie2-Cre-loxP-GFP reporter mice. (E) Comparative G-csf transcript levels normalized to Actb in sorted CD45−Ter119−GFP+CD31+ ECs (red bars) vs CD45−Ter119−GFP+CD31− non-ECs (white bars) isolated from BM, heart, liver, kidney, spleen, and lung of LPS-injected Tie2-Cre-loxP-GFP reporter mice. All data represent mean ± standard deviation from 2 independent experiments (n = 5 mice). Two-tailed Student t tests were used to assess statistical significance (*P < .05, **P < .01, ***P < .001).
To evaluate the role of these CD45−Ter119−GFP+CD31− cells for the initiation of emergency granulopoiesis, we injected Tie2-Cre-loxP-GFP reporter mice with LPS, sorted the CD31+ EC and CD31− non-EC fractions of the CD45−Ter119−GFP+ nonhematopoietic compartment and compared G-csf expression by quantitative reverse-transcription PCR as a surrogate marker for the ability of the respective cell type to stimulate emergency granulopoiesis (Figure 4A). There was no difference in steady-state G-csf expression between both cell fractions (data not shown), whereas after LPS injection, G-csf expression in CD45−Ter119−GFP+CD31+ ECs was always significantly higher (2- to 5-fold) than in the CD45−Ter119−GFP+CD31− non-EC fraction in all organs analyzed (Figure 4E). Thus, given the 4-fold higher numbers of CD45−Ter119−GFP+CD31+ ECs vs CD45−Ter119−GFP+CD31− non-ECs and the 2- to 5-fold higher amount of G-csf expression in CD45−Ter119−GFP+CD31+ ECs vs CD45-Ter119−GFP+CD31− non-ECs, we infer that most (>95%) of G-CSF derived from ECs and, importantly, Tie2-Cre mice, are a valid tool to assess ECs within the nonhematopoietic compartment.
Collectively, ECs are equipped with the functional machinery to detect LPS and are indeed the dominant G-CSF–producing cell population upon systemic LPS challenge, sufficient to induce emergency granulopoiesis.
EC-intrinsic MYD88 signaling is required to stimulate accelerated neutrophil production, myeloid progenitor lineage skewing toward granulocyte-macrophage progenitors, and increased CFU-G activity in vivo
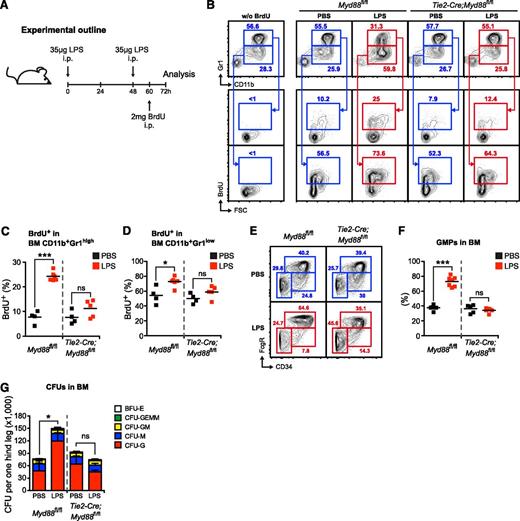
Next, we assessed neutrophil generation in the BM of Myd88fl/fl and Tie2-Cre;Myd88fl/fl mice in the steady state and during LPS-induced emergency granulopoiesis (Figure 5A). During 12 hours, approximately 10% and 50% of BM CD11b+Gr1high mature and BM CD11b+Gr1low immature neutrophils incorporated BrdU into newly synthesized DNA in PBS-injected control and Tie2-Cre;Myd88fl/fl mice, respectively (Figure 5B-D). After LPS injection, there was a significant increase in BrdU incorporation in Myd88fl/fl mice in both BM CD11b+Gr1high mature and BM CD11b+Gr1low immature neutrophils. However, this increase was absent in LPS-injected Tie2-Cre;Myd88fl/fl mice (Figure 5B-D). These results demonstrate that neutrophil turnover is significantly accelerated upon LPS stimulation, and that this response is dependent on EC-intrinsic MYD88 signaling.
Endothelial cell–intrinsic MYD88 signaling is required to stimulate accelerated neutrophil production, myeloid progenitor lineage skewing toward granulocyte-macrophage progenitors (GMPs), and increased CFU-G activity in vivo. (A) Graphical scheme depicting experimental outline to induce LPS-induced emergency granulopoiesis and to assess BrdU incorporation. (B) Representative FACS profile showing BrdU incorporation in in BM CD11b+Gr1high mature and BM CD11b+Gr1low immature neutrophils in control Myd88fl/fl, and Tie2-Cre;Myd88fl/fl mice during steady-state and LPS-induced emergency granulopoiesis. (C) Frequencies of BrdU+ BM CD11b+Gr1high mature and (D) BrdU+ BM CD11b+Gr1low immature neutrophils in PBS- or LPS-injected Myd88fl/fl and Tie2-Cre;Myd88fl/fl mice. (E) Representative FACS profile showing myeloerythroid progenitors in control Myd88fl/fl, and Tie2-Cre;Myd88fl/fl mice during steady-state and LPS-induced emergency granulopoiesis. (F) Frequencies of Lin−cKit+Sca1−FcgR+CD34+ GMPs in PBS- or LPS-injected Myd88fl/fl and Tie2-Cre;Myd88fl/fl mice. (G) Absolute CFU numbers per 1 hind leg in control Myd88fl/fl and Tie2-Cre;Myd88fl/fl mice during steady-state and LPS-induced emergency granulopoiesis (CFU-G, CFU granulocyte; CFU-M, CFU macrophage; CFU-GM, CFU granulocyte/macrophage; CFU-GEMM, CFU granulocyte/erythrocyte/macrophage/megakaryocyte; BFU-E, burst-forming unit erythrocyte). Black squares, PBS-injected mice; red squares, LPS-injected mice. Data from 2 independent experiments are shown. Two-tailed Student t tests were used to assess statistical significance (*P < .05, ***P < .001).
Endothelial cell–intrinsic MYD88 signaling is required to stimulate accelerated neutrophil production, myeloid progenitor lineage skewing toward granulocyte-macrophage progenitors (GMPs), and increased CFU-G activity in vivo. (A) Graphical scheme depicting experimental outline to induce LPS-induced emergency granulopoiesis and to assess BrdU incorporation. (B) Representative FACS profile showing BrdU incorporation in in BM CD11b+Gr1high mature and BM CD11b+Gr1low immature neutrophils in control Myd88fl/fl, and Tie2-Cre;Myd88fl/fl mice during steady-state and LPS-induced emergency granulopoiesis. (C) Frequencies of BrdU+ BM CD11b+Gr1high mature and (D) BrdU+ BM CD11b+Gr1low immature neutrophils in PBS- or LPS-injected Myd88fl/fl and Tie2-Cre;Myd88fl/fl mice. (E) Representative FACS profile showing myeloerythroid progenitors in control Myd88fl/fl, and Tie2-Cre;Myd88fl/fl mice during steady-state and LPS-induced emergency granulopoiesis. (F) Frequencies of Lin−cKit+Sca1−FcgR+CD34+ GMPs in PBS- or LPS-injected Myd88fl/fl and Tie2-Cre;Myd88fl/fl mice. (G) Absolute CFU numbers per 1 hind leg in control Myd88fl/fl and Tie2-Cre;Myd88fl/fl mice during steady-state and LPS-induced emergency granulopoiesis (CFU-G, CFU granulocyte; CFU-M, CFU macrophage; CFU-GM, CFU granulocyte/macrophage; CFU-GEMM, CFU granulocyte/erythrocyte/macrophage/megakaryocyte; BFU-E, burst-forming unit erythrocyte). Black squares, PBS-injected mice; red squares, LPS-injected mice. Data from 2 independent experiments are shown. Two-tailed Student t tests were used to assess statistical significance (*P < .05, ***P < .001).
We also analyzed the myeloid progenitor compartment in steady-state and LPS-injected Myd88fl/fl and Tie2-Cre;Myd88fl/fl mice. In accordance with previously published results,44 we observed an increase in the percentage of Lin−cKit+Sca1−FcgR+CD34+ granulocyte-macrophage progenitors (GMPs) upon LPS stimulation in Myd88fl/fl mice. This response was absent in Tie2-Cre;Myd88fl/fl mice (Figure 5E-F). These data demonstrate that LPS induces a lineage bias at the HPC level in favor of enhanced granulopoiesis.
Finally, we determined the CFU activity in BM from steady-state or LPS-injected Myd88fl/fl and Tie2-Cre;Myd88fl/fl mice. After LPS injection, there was a significant increase in the overall number of CFUs in Myd88fl/fl mice. Notably, this increment was due to an increase in CFU-granulocyte (CFU-G) with no changes in the number of other CFU types. Importantly, this response was absent in LPS-treated Tie2-Cre;Myd88fl/fl mice (Figure 5G).
EC-intrinsic MYD88 signaling is required to efficiently stimulate emergency granulopoiesis during systemic E coli infection
To evaluate whether MYD88 signaling in ECs is also important to initiating emergency granulopoiesis in a far more complex setting of infection with living bacteria, we performed experiments with E coli. First, we titrated the E coli dose necessary to elicit a similar quantitative response in WT mice as observed with the LPS dose used in prior experiments. We determined this dose to be 4.5 × 108E coli CFU given IP per WT mouse (supplemental Figure 3A-E). In parallel, we also infected Myd88−/− mice. Although these mice showed some response to E coli, the responses were severely and highly significantly reduced compared with WT mice for the entire previously assessed readouts characteristic for emergency granulopoiesis (supplemental Figure 3A-E). Importantly, the minor MYD88-independent responses upon E coli infection are also independent of G-CSF because there was no significant rise in plasma G-CSF levels in Myd88−/− mice (supplemental Figure 3E).
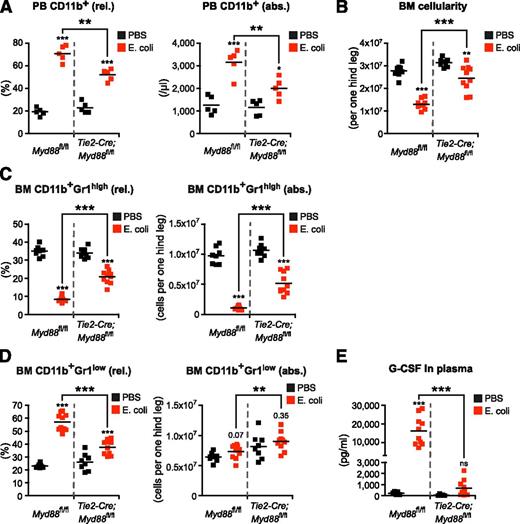
Next, we infected control Myd88fl/fl and Tie2-Cre;Myd88fl/fl mice with E coli (4.5 × 108 CFU per mouse). We observed the same pattern, with Myd88fl/fl mice showing the same response as WT mice, and Tie2-Cre;Myd88fl/fl mice showing a minor, in comparison with Myd88fl/fl mice significantly reduced response. This indicates that EC-intrinsic MYD88 signaling is required to induce full-blown emergency granulopoiesis also upon live E coli infection (Figure 6A-E). The minor response observed in Tie2-Cre;Myd88fl/fl mice in an infection with live bacteria might not be surprising because in this far more complex situation, in contrast to exclusive LPS-mediated stimulation, other pattern-recognition receptor agonists and additional tissue damage also might activate alternative, independent signaling pathways. Notably, however, plasma G-CSF levels did not rise above steady-state levels in Tie2-Cre;Myd88fl/fl mice, revealing ECs to be major sources of G-CSF also during infection with E coli (Figure 6E).
Endothelial cell–intrinsic MYD88 signaling is required to efficiently stimulate emergency granulopoiesis during systemic E coli infection. (A) Frequency and absolute number of PB CD11b+ cells, (B) BM cellularity, (C) frequencies and absolute numbers of BM CD11b+Gr1high mature and (D) BM CD11b+Gr1low immature neutrophils, and (E) plasma G-CSF levels in Myd88fl/fl mice and Tie2-Cre;Myd88fl/fl mice in steady-state (black squares) and after infection with 4.5 × 108E coli CFU (red squares) given IP. Data from 2 independent experiments are shown. rel., relative; abs., absolute. Two-tailed Student t test was used to assess statistical significance (**P < .001, ***P < .001).
Endothelial cell–intrinsic MYD88 signaling is required to efficiently stimulate emergency granulopoiesis during systemic E coli infection. (A) Frequency and absolute number of PB CD11b+ cells, (B) BM cellularity, (C) frequencies and absolute numbers of BM CD11b+Gr1high mature and (D) BM CD11b+Gr1low immature neutrophils, and (E) plasma G-CSF levels in Myd88fl/fl mice and Tie2-Cre;Myd88fl/fl mice in steady-state (black squares) and after infection with 4.5 × 108E coli CFU (red squares) given IP. Data from 2 independent experiments are shown. rel., relative; abs., absolute. Two-tailed Student t test was used to assess statistical significance (**P < .001, ***P < .001).
To test whether TLR adaptor TRIF might partially compensate for the severely diminished E coli–induced emergency granulopoiesis in Myd88−/− and Tie2-Cre;Myd88fl/fl mice, and to determine the relative contribution of each pathway to it, we infected WT, Tlr4−/−, Myd88−/−, and Trif−/− mice with 4.5 × 108E coli CFU IP. We observed that both Tlr4−/− and Myd88−/− mice show an equally reduced ability to launch emergency granulopoiesis compared with WT mice. By stark contrast, Trif−/− mice responded to E coli (supplemental Figure 4) with emergency granulopoiesis, as did WT mice. These data demonstrate that TRIF is dispensable for the initiation of emergency granulopoiesis.
In summary, our findings demonstrate that abrogated MYD88 signaling in ECs leads to severely defective emergency granulopoiesis upon E coli infection.
Discussion
We addressed here the fundamental question of which cell types act as primary sensors of bacterial dissemination during severe infection and consequently induce the switch from steady-state to demand-adapted emergency granulopoiesis.
Two general models have been proposed for how pathogen sensing and translation into emergency granulopoiesis may be achieved.7,8 According to the concept of indirect hematopoietic activation, tissue-resident macrophages might serve in this critical function46,47 because they indeed express pattern-recognition receptors, and, after respective stimulation, produce granulopoietic growth factors.48-51 But data from stringent in vivo experimentation supporting this model are lacking. Alternatively, several recent studies suggested a model of direct hematopoietic activation based on the observation that mouse and human HSPCs express TLRs and that TLR agonist stimulation leads to enhanced myelopoieisis.16-19,21 However, these studies used either in vitro16 or very specific in vivo experiments such as adoptive HSPC transfer under the renal capsule,18 direct injection of HSPCs into Staphylococcus aureus–infected wounds,21 or pretreatment with chemotherapeutic agents and ionizing radiation22 to convey their respective proposed model. By contrast, we have demonstrated that pathogen sensing by both immature HSPCs and mature hematopoietic cells, including macrophages, is dispensable for the acute process of emergency granulopoiesis in 3 independent and complementary experimental approaches. First, in both hematopoietic Tlr4−/− chimeric mice23 and hematopoietic Myd88−/− chimeric mice, emergency granulopoiesis is indistinguishable from that in WT mice. Of note, we and others could show that BM chimeric mice are an appropriate tool to broadly dissect biological effects contributed by either hematopoietic or nonhematopoietic tissues by confirming that tissue-resident macrophages, although they are able to locally self-renew in the steady state,52 are replenished by donor-derived cells after lethal irradiation.23,52 Second, liposomal clodronate–mediated macrophage-depleted WT mice launch undiminished emergency granulopoiesis upon LPS stimulation.23 Third, as shown here, mice with a Myd88-deficiency in the myeloid compartment, most importantly macrophages (LysM-Cre;Myd88fl/fl mice), respond normally toward LPS with emergency granulopoiesis.
To determine the identity of the nonhematopoietic cell types required for the initiation of emergency granulopoiesis, we undertook an extensive genetic targeting approach using Cre recombinase–mediated tissue-specific Myd88 ablation. Specifically, we targeted the nonhematopoietic BM microenvironment (Nestin+ mesenchymal stem cells and perivascular stromal cells) using Nes-Cre36-38 and Pdgfrb-Cre39 mice. These cells have been shown to be part of the hematopoiesis-supporting BM microenvironment.53 Vascular cells (ECs and pericytes) were targeted using Tie2-Cre37,38,40,54 and Pdgfrb-Cre39 mice, respectively. In addition, we used Alb-Cre41 mice to target hepatocytes as a candidate prototypic parenchymal cell type. Our data revealed that ECs express high amounts of Tlr4 and Myd88 and respond to systemic LPS stimulation or E coli infection with massive upregulation of the primary granulopoiesis-supporting growth factor G-CSF. Most importantly, mice with a Myd88 deficiency in ECs do not respond with emergency granulopoiesis upon systemic LPS stimulation and have a severely defective response toward E coli infection. The E coli–driven MYD88- and G-CSF–independent minor responses in Myd88−/− and Tie2-Cre;Myd88fl/fl mice are likely triggered by activation of alternative, redundant pathways that evolved to secure this critical response. Of interest, a recent study showed that granulopoiesis after antibody-mediated neutrophil removal depends on TLR4/TRIF signaling.55 We therefore studied whether a similar feedback regulatory mechanism via TLR4/TRIF might account for the residual emergency granulopoiesis observed in E coli–infected Myd88−/− and Tie2-Cre;Myd88fl/fl mice. However, we observed that TLR4 signal transduction via TRIF is dispensable for the induction of E coli–driven emergency granulopoiesis as Trif−/− mice show a response that is indistinguishable from those in WT mice.
Because congenital33 or iatrogenic32 forms of neutropenia are associated with a high incidence of lethal infectious complications, it seems very likely that the ability to induce emergency granulopoiesis per se has a live-saving role in massive infection. However, direct experimental evidence for this notion is lacking and future studies will need to dissect emergency granulopoiesis from neutrophil recruitment to inflamed tissues and neutrophil effector functions, which together ensure host survival during bacterial infection.
Given the general caveat of lack of absolute tissue specificity in the Cre-loxP system, we have analyzed Tie2-Cre-loxP-GFP reporter mice. In accordance with the literature,37,38,40,54 Tie2-Cre mice are an excellent tool to genetically manipulate ECs, but, of note, there is also a small fraction of other (ie, non-EC and nonhematopoietic) cells targeted in Tie2-Cre mice. However, we have addressed this issue experimentally by analyzing the G-CSF response in non-ECs in these mice and could exclude a relevant contribution of these nonhematopoietic, non-EC cell populations to the overall emergency granulopoiesis response.
Interestingly, the versatile and critical function of endothelium is an emerging aspect in hematopoiesis research. It has recently been demonstrated that ECs are an important constituent of the BM microenvironment. Using a similar genetic tissue-specific deletion approach, ECs were shown to be major sources of stem cell factor37 and CXCL12,38,54 2 molecules that are involved in HSPC maintenance and their BM retention, respectively. Our results now show that ECs are not only critical for the regulation of steady-state hematopoietic maintenance but are also essential for demand-adapted hematopoiesis in response to the paradigmatic gram-negative bacteria–derived compound LPS and the clinically relevant pathogen E coli. In conjunction with previously published data on the importance of TLR signaling in ECs for neutrophil recruitment to inflamed tissues,14,15 our findings reveal a sophisticated degree of functional interplay between the vascular, the hematopoietic, and the immune systems, which have a common developmental root and might thus be understood as 1 functional organ.56,57 Of note, our findings also confirm and extend prior landmark observations on the importance of nonhematopoietic cell-derived hematopoietic growth factors.58,59 Although these seminal studies established a role for nonhematopoietic cells as a major source of hematopoietic growth factors, they, because of technical limitations at that time, were unable to identify the precise identity of this cell type that we now show to be ECs.
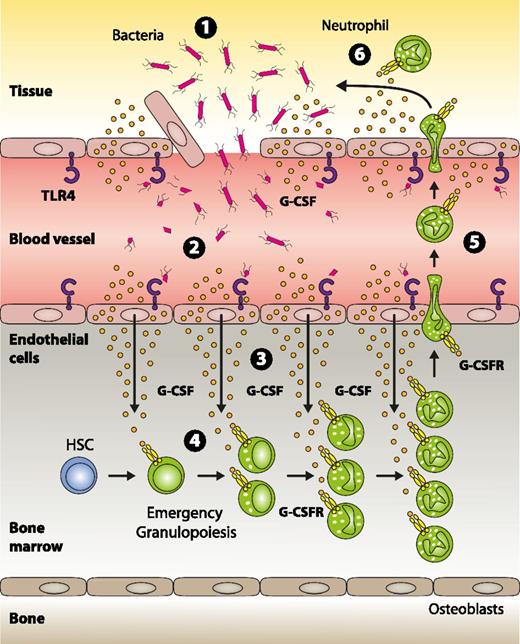
ECs are ideally positioned to mark the threshold of local vs systemic infection, and using ECs as safeguards seems a very plausible, danger-adapted defense strategy established in evolution. In summary, based on the data presented here, we propose a model (Figure 7) in which ECs are critical for pathogen sensing and subsequent induction of emergency granulopoiesis.
Model for pathogen sensing and subsequent translation into emergency granulopoiesis. (1) Gram-negative bacteria and/or their structural components that have gained access to the systemic circulation are recognized by TLR4-expressing endothelial cells (2), thereby indicating an emergency state. Upon TLR4/MYD88 signaling in endothelial cells, G-CSF is released in large quantities (3). In the bone marrow, endothelial cell–derived G-CSF acts on myeloid precursors expressing the G-CSF receptor resulting in enhanced generation, accelerated turnover, and increased neutrophil release from the bone marrow to the systemic circulation (4). These neutrophils are recruited to the site of infection (5) where they participate in clearing the pathogen (6).
Model for pathogen sensing and subsequent translation into emergency granulopoiesis. (1) Gram-negative bacteria and/or their structural components that have gained access to the systemic circulation are recognized by TLR4-expressing endothelial cells (2), thereby indicating an emergency state. Upon TLR4/MYD88 signaling in endothelial cells, G-CSF is released in large quantities (3). In the bone marrow, endothelial cell–derived G-CSF acts on myeloid precursors expressing the G-CSF receptor resulting in enhanced generation, accelerated turnover, and increased neutrophil release from the bone marrow to the systemic circulation (4). These neutrophils are recruited to the site of infection (5) where they participate in clearing the pathogen (6).
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ralf H. Adams (Max Planck Institute for Molecular Biomedicine, Muenster, Germany) for providing Pdgfrb-Cre mice.
This work was supported by the Swiss National Science Foundation (310030_146528/1), the Promedica Foundation (Chur, Switzerland), and the Clinical Research Priority Program of the University of Zurich (M.G.M.).
Authorship
Contribution: S.B. devised, performed and analyzed experiments, and wrote the manuscript; R.C.G., R.R., J.B., and F.A. performed experiments; M.H. and M.K. devised experiments and discussed data; and M.G.M. directed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markus G. Manz, Division of Hematology / University Hospital Zurich, Raemistrasse 100, CH-8091 Zurich, Switzerland; e-mail: markus.manz@usz.ch.