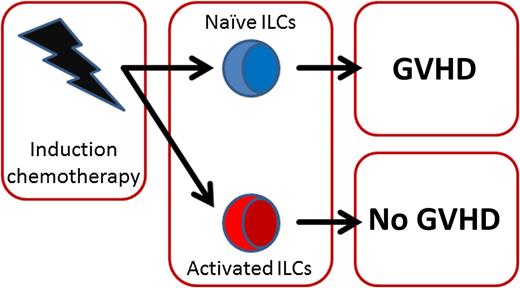
Activated ILCs are correlated with less GVHD. After induction chemotherapy, prior to HSCT, most ILCs are depleted. However, the remaining ILCs can have either an activated or naïve phenotype. Patients with mostly activated ILCs had a significantly lower incidence of GVHD after transplant.
Activated ILCs are correlated with less GVHD. After induction chemotherapy, prior to HSCT, most ILCs are depleted. However, the remaining ILCs can have either an activated or naïve phenotype. Patients with mostly activated ILCs had a significantly lower incidence of GVHD after transplant.
ILCs are a diverse family of lymphocytes whose ability to influence tissue homeostasis makes them prime candidates to regulate human disease. However, most of our knowledge on ILCs still stems from studies in experimental mouse models.
Hazenberg and Spits elegantly explain that human ILCs exist as functionally distinct subsets that mirror T helper cell specification.1 In short, ILC1 secrete interferon γ (IFNγ) and are involved in intestinal inflammation, ILC2 function during type 2 immune responses to extracellular pathogens and helminthes through production of interleukin-13 (IL-13) and IL-5 and were implicated in tissue repair in the lung, and ILC3 that produce IL-22 and IL-17 are important in defense against enteric pathogens by activating epithelial cells.3 The major difference with T helper cells is that ILCs lack an antigen receptor and are activated either by cytokines or by microbial patterns recognized through Toll-like receptors.4 ILCs are present in the human intestine and skin, where they can be activated by commensal microbiotas.5,6
The development of acute GVHD remains one of the major limitations in the efficacy of HSCT. HSCT is a powerful treatment option for hematologic malignancies. HSCT is often curative and for some diseases HSCT is the only curative treatment available.7 Since the implementation of HSCT in the 1960s, the occurrence of adverse and potentially fatal reactions of the infused cells to the host have been a major clinical problem. Over the years, depletion of the allogeneic effector T cells from the graft and the introduction and refinement of immunosuppressive therapies for all transplant patients have significantly reduced the occurrence of acute GVHD.8 However, still there is insufficient knowledge on the pathobiology of GVHD initiation to allow for the prospective identification of those patients that will develop acute GVHD after transplant, let alone to be able to prevent acute GVHD from developing.
One of the earliest events in the cascade leading to GVHD is tissue damage inflicted by induction chemotherapy preceding HSCT. Chemotherapy damages the epithelia that line the mucosal barriers of the skin and the gastrointestinal (GI) tract. Disrupted barrier function leads to translocation of commensal microbiotas, present on the skin and in the GI tract, which fuels continuous activation of innate immune cells creating and maintaining an inflammatory milieu permissive for subsequent activation of deleterious adaptive immunity.9 Minimizing tissue damage or maximizing tissue repair are considered promising strategies to limit GVHD development.
In an elegant study, Munneke et al now show that, in acute myeloid leukemia patients, ILC numbers in the peripheral blood are strongly reduced following induction therapy prior to HSCT. Even so, natural cytotoxicity receptor (NCR)+ ILCs, which have been linked to intestinal homeostasis in animal models, are absent from the circulation in healthy controls but appear in patients after induction therapy.2 This suggests that the increase in this population might be driven by signals related to tissue damage or the conditioning regimen, a notion that is supported by experimental data in a mouse model of HSCT.10
More importantly, the authors noted a clear dichotomy in the ILCs present after induction therapy. In a subgroup of patients, ILCs had an activated phenotype and expressed molecules involved in homing to the intestine. In other patients, ILCs appeared mostly naïve and lacked expression of these homing integrins. Interestingly, the group of patients that had activated ILCs prior to transplantation were less likely to develop GVHD after HSCT2 (see figure). These data suggest that activation of ILCs as a result of pretransplant induction therapy might minimize subsequent GVHD development.
After transplantation, ILC recovery was slow when compared with neutrophils and monocytes. At 7 weeks posttransplant, all host ILCs had disappeared and were replaced by donor-derived cells. This notwithstanding, the composition of the ILC subsets was still altered compared with healthy controls and the characteristic presence of the tissue-damage related NCR+ ILCs noted after induction therapy was still apparent.
Future studies will have to focus on determining whether the correlation found by Munneke et al between ILC activation status and absence of GVHD reflects a protective effect of ILCs. If ILCs are capable of protecting from GVHD development, it is likely that this will be through their ability to enhance tissue repair, as was shown for experimental models of influenza-induced lung damage.11 Reduced tissue damage would restrict bacterial translocation and innate immune activation, dampening GVHD development at a very early stage. If so, this would allow for the design of new therapeutic strategies to prevent GVHD by activating ILC in situ or, more likely, through exogenous administration of ILC-derived molecules involved in mucosal healing. Alternatively, ILC activation status, or the levels of signature cytokines related to this activation by these cells, might be able to serve as a predictor for the development of acute GVHD.
Conflict-of-interest disclosure: The author declares no competing financial interests.