To the editor:
The Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib sets a new standard of care for the treatment of patients with relapsed or refractory chronic lymphocytic leukemia (CLL)1,2 and mantle cell lymphoma (MCL).3 Ibrutinib is generally well tolerated and largely devoid of side effects such as marrow suppression and excess infections.1-3 However, atrial fibrillation (AF) is emerging as a potentially therapy-limiting adverse effect of ibrutinib, occurring in 3.5% to 6.5% of subjects across multiple trials in CLL, MCL, and Waldenstrom macroglobulinemia (summarized in supplementary Table 1, available on the Blood Web site).1-6 This excess risk is most evident in the randomized RESONATE study comparing ibrutinib with ofatumumab, in which there was a 10-fold increased rate of AF in the ibrutinib arm.2 Understanding the mechanisms underlying ibrutinib-associated AF and identifying potential risk factors and management strategies are crucial for the widespread safe use of this drug in the community.
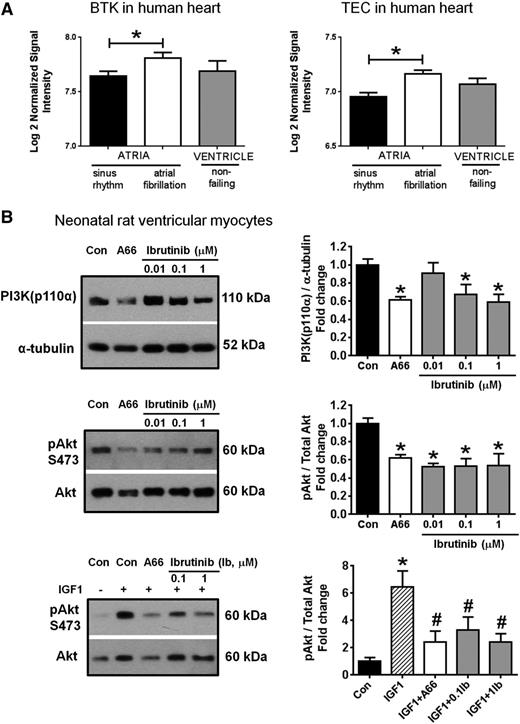
We hypothesize that ibrutinib-induced AF may be due to on-target inhibition of BTK and related kinases such as tec protein tyrosine kinase (TEC), which hitherto were not known to have functions in the human heart. First, we mined publically available microarray data at NCBI’s Gene Expression Omnibus for studies assessing gene expression in human heart tissue. On analyzing data from a study comparing cardiac messenger RNA expression in patients in permanent AF to patients in sinus rhythm (accession number GSE22407 ; http://www.ncbi.nlm.nih.gov/geo/), we showed that both BTK and TEC transcripts were expressed in human heart tissue (Figure 1A). Moreover, the expressions of BTK and TEC transcripts were higher in atrial tissue under conditions of AF than sinus rhythm (Figure 1A) (P < .05), suggesting that BTK and TEC may have functional roles under conditions of cardiac stress.
Expression of BTK and TEC in the human heart, and the effect of ibrutinib on activation of the PI3K-Akt pathway in NRVM. (A) Gene expression of BTK and TEC in human atrial and ventricular tissue. Data are expressed as mean ± standard error of the mean. Atrial samples: sinus rhythm (N = 20), AF (N = 10); ventricle (N = 5). *P < .05. Unpaired Student t-test. Data obtained from microarray deposited at http://www.ncbi.nlm.nih.gov/geo/, accession number GSE2240. (B) The effect of ibrutinib treatment on PI3K(p110α) protein expression and/or pAkt/total Akt in NRVM under (1) basal conditions (top 2 panels), and (2) in the presence of insulin-like growth factor 1 (IGF1, 10 nM, +, bottom panel). A66 (10 µM), specific inhibitor of PI3K(p110α), was administered alone (top 2 panels) or together with IGF1 (bottom panel). Data are expressed as mean ± standard error of the mean of triplicates (under basal conditions, results are representative of 3 independent NRVM preparations). *P < .05 vs control (Con); #P < .05 vs IGF1. Analysis of variance followed by Fisher’s post-hoc tests. Ib, ibrutinib.
Expression of BTK and TEC in the human heart, and the effect of ibrutinib on activation of the PI3K-Akt pathway in NRVM. (A) Gene expression of BTK and TEC in human atrial and ventricular tissue. Data are expressed as mean ± standard error of the mean. Atrial samples: sinus rhythm (N = 20), AF (N = 10); ventricle (N = 5). *P < .05. Unpaired Student t-test. Data obtained from microarray deposited at http://www.ncbi.nlm.nih.gov/geo/, accession number GSE2240. (B) The effect of ibrutinib treatment on PI3K(p110α) protein expression and/or pAkt/total Akt in NRVM under (1) basal conditions (top 2 panels), and (2) in the presence of insulin-like growth factor 1 (IGF1, 10 nM, +, bottom panel). A66 (10 µM), specific inhibitor of PI3K(p110α), was administered alone (top 2 panels) or together with IGF1 (bottom panel). Data are expressed as mean ± standard error of the mean of triplicates (under basal conditions, results are representative of 3 independent NRVM preparations). *P < .05 vs control (Con); #P < .05 vs IGF1. Analysis of variance followed by Fisher’s post-hoc tests. Ib, ibrutinib.
One of the pathways regulated by BTK and TEC is the phosphoinositide 3-kinase (PI3K)-Akt pathway. This pathway is a critical regulator of cardiac protection under stress conditions.8 Our group showed that mice with reduced cardiac PI3K-Akt activity were highly susceptible to AF,9 and that these observations were relevant in humans, as surgical specimens from patients with AF showed significantly lower cardiac PI3K-Akt activity than those from patients in sinus rhythm.9 The cardioprotective effect of this pathway is further underscored by our observation that augmentation of PI3K-Akt activity in murine models of heart failure improved the function of the failing heart.8,10 In view of the unexplained occurrence of AF in ibrutinib-treated patients, we sought to determine if ibrutinib impacts on PI3K-Akt signaling in an experimental model.
Neonatal rat ventricular myocytes (NRVM) were exposed to ibrutinib (0.01-1 μM) under (1) basal conditions for 48 hours and (2) in the presence of stimulation by insulin-like growth factor 1 ([IGF1], 10 nM, upstream activator of PI3K) for 18 hours. As a positive control, a specific inhibitor of PI3K(p110α), A66 (10 µM), was also administered alone or together with IGF1. PI3K(p110α) protein expression and/or Akt activation (pAkt/total Akt; key downstream target of PI3K) was then examined by western blot analysis. In these experiments, we observed reduced PI3K(p110α) protein expression and/or Akt activation (pAkt/total Akt) in NRVM treated with ibrutinib under basal conditions (Figure 1B, top panels). Moreover, exposure to ibrutinib attenuated the Akt response after PI3K(p110α) stimulation by IGF1 (Figure 1B, bottom panel). Taken together, our findings indicate that ibrutinib significantly reduced PI3K-Akt activity in cardiac cells at 0.1 to 1 µM concentration. As a point of reference, the mean peak plasma concentration of ibrutinib in humans at clinical doses is approximately 0.3 µM.
These observations highlight the inhibition of protective PI3K-Akt signaling as one potential explanation for AF occurrence in ibrutinib-treated patients. Crucially, the PI3K-Akt pathway is also important for prevention of stress-induced cardiomyopathy,8,10 a complication that has not been reported to date in ibrutinib clinical studies.1-6 We recommend that clinicians remain vigilant for the emergence of AF and other unexplained cardiac complications in patients treated with ibrutinib and other agents that impact on the PI3K-Akt pathway, particularly those which inhibit PI3K(p110α) (the dominant cardioprotective PI3K isoform). Further mechanistic studies to comprehensively assess the impact of these drugs on electrocardiographic parameters, cardiac function, and PI3K-Akt signaling are warranted.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: This work was supported by the authors’ institutions, which had no role in the design of the experiments or the writing of the paper.
Contribution: J.R.M. performed analysis and interpretation, and wrote the paper; J.R.M., C.S.T., J.F.S., and M.J.K. designed the study and wrote the paper; and E.J.H.B. and J.Y.Y.O. performed laboratory work and data analysis, and approved the paper.
Conflict-of-interest disclosure: C.S.T. receives honorarium from Pharmacyclics and Janssen-Cilag, and J.F.S. is a member of advisory boards and receives honorarium from Janssen-Cilag. The remaining authors declare no competing financial interests.
Correspondence: Constantine S. Tam, Haematology Department, Peter MacCallum Cancer Center, St. Andrew’s Place, East Melbourne, Victoria, Australia; e-mail: constantine.tam@petermac.org.