In this issue of Blood, de Stoppelaar et al further unravel the relevance of platelets in a mouse model of pneumonia-derived sepsis by illustrating how platelets dynamically modulate infection and the inflammatory response.1
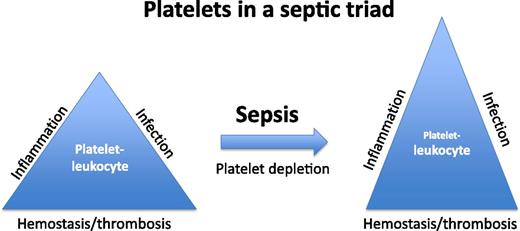
Platelets are increasingly recognized as participating in the inflammatory response in addition to their well-characterized role in hemostasis and thrombosis. Depicted in a conceptual triangle is a platelet-leukocyte balance supporting normal homeostasis. In the condition of sepsis, significant dysregulation of a platelet-leukocyte balance occurs. In the study by de Stoppelaar et al,1 the significance of the platelet is further defined in septic animals with experimentally induced thrombocytopenia. The dysregulation of the septic triad leads to an additional imbalance between infection, inflammation, and hemostasis/thrombosis.
Platelets are increasingly recognized as participating in the inflammatory response in addition to their well-characterized role in hemostasis and thrombosis. Depicted in a conceptual triangle is a platelet-leukocyte balance supporting normal homeostasis. In the condition of sepsis, significant dysregulation of a platelet-leukocyte balance occurs. In the study by de Stoppelaar et al,1 the significance of the platelet is further defined in septic animals with experimentally induced thrombocytopenia. The dysregulation of the septic triad leads to an additional imbalance between infection, inflammation, and hemostasis/thrombosis.
A major challenge in sepsis research is understanding the complex interplay of thrombotic and inflammatory processes that lead to a widespread vascular collapse and death. Although strong individual paradigms exist for describing clotting and inflammation, when and how these processes influence each other is less clear. Thrombocytopenia is a common finding in severe sepsis and is increasingly recognized as more than just a simple marker of consumptive coagulopathy.2 Indeed, together with other studies, the work of de Stoppelaar et al suggests that circulating platelets promote inflammation during the early stages of infection, but later inhibit cytokine release when the inflammatory response becomes high.
Beyond the mechanistic complexities of sepsis are the limitations imposed by various animal models and the ability to translate findings to the human condition. Several options exist, and de Stoppelaar et al have used a mouse model of Klebsiella infection, a common respiratory pathogen and causative agent for sepsis in pneumonia. The investigators chose to deplete platelets in animals to varying degrees by using antiplatelet antibodies and then evaluate in vivo parameters such as survival, bacterial growth, hemorrhage, distant organ damage, and proinflammatory cytokine release.
To fully appreciate the findings of de Stoppelaar et al requires a detailed consideration for the temporal sequence of events that lead to the septic condition. One of the simplest conclusions of their study is that after platelet depletion and inoculation with Klebsiella, serum markers of inflammation such as tumor necrosis factor (TNF)-α and interleukin-6 (IL-6) are elevated, and animal survival is decreased. This data alone would suggest that normal platelet levels are important for survival and for impairing cytokine release. However, during the earliest stages of infection, platelet depletion increases the bacterial load in the bloodstream, which suggests a platelet-dependent mechanism in the innate immune response. This indicates that platelets participate in a protective manner in early sepsis by lowering the bacterial load, and then in the later stages of sepsis, they modulate cytokine levels during the “cytokine storm.” Thus, the platelet has a central role in modulating the balance between antimicrobial response, inflammatory response, and hemostasis/thrombosis (see figure).
The molecular basis for how platelets participate in the pathophysiology of sepsis is still to be defined. An initial, perhaps overly simplistic, thought is to attribute the platelet-dependent consequences during sepsis to circulating and intact platelets. However, platelet activation and the release of platelet microparticles may be more relevant mediators of the pathophysiology associated with sepsis.3 Work by Xiang et al4 supports the relevance of platelets in a lipopolysaccharide (LPS)-induced endotoxemia and a bacterial infusion mouse sepsis model while implicating activation of a platelet COX1 pathway in regulating the inflammatory response. The platelet adhesion receptor glycoprotein Ib-IX (GPIb-IX) has also been implicated in the regulation of the immune response in models of both LPS and polymicrobial peritonitis.5,6 GPIb-IX–dependent platelet interactions with neutrophils and monocytes were also documented to influence neutrophil and monocyte phenotype.6 Going forward, a more comprehensive view of the molecular interactions between platelets and immune cells is needed. This will require establishing models that allow dissecting the platelet’s role in hemostasis, thrombosis, and inflammation.
There have been major criticisms for how closely any murine models of sepsis mimic the human septic situation. Indeed, every model has its limitations, and the human septic situation is a heterogeneous clinical situation that results from differences in the primary sites of infection, the pathogens involved, and the overall health status of the patient. Some have even suggested that studies of inflammation in murine models have no merit because of observed differences in global gene expression patterns during infection or inflammation when comparing mice and humans.7 Recently however, others have argued the opposite, suggesting that mouse models are an important tool for modeling inflammatory diseases.8 Independently, both hemostasis/thrombosis and inflammation have been modeled for decades by using rodent models with significant impact on and relevance to our understanding of human pathophysiology. Thus, mouse models are likely to continue as a major experimental approach to the topic of sepsis for generating new hypotheses that can be further evaluated for the clinically challenging topic of human sepsis.
To the practicing physician, and even more so the practicing hematologist, defining the relevance of platelets in the inflammatory events occurring in the progression of sepsis is likely to be important for guiding patients in their use of antiplatelet medications. Important questions need to be addressed. How relevant are the major antiplatelet medications, such as aspirin, in the progression of sepsis? Are the molecular events associated with sepsis simply too robust for any antiplatelet medication to impact the course of the disease? Is targeting platelet-leukocyte interactions a potential therapeutic approach in sepsis? Although these questions are far beyond the implications of the work by de Stoppelaar et al in this issue of Blood, they do remind us of the platelet’s exclusive presence in mammals. How much relevance to inflammation has been retained from its counterpart cell—the thrombocyte—in lower vertebrates? Although the platelet has been the cornerstone of research in thrombosis, adapting platelet paradigms to bridge hemostasis, thrombosis, and inflammation marks a major challenge in platelet research going forward. More studies, such as those reported in this issue of Blood, are needed and are likely to have major impacts in our understanding and therapeutic approaches to address unmet clinical needs.
Conflict-of-interest disclosure: The authors declare no competing financial interests.