Key Points
Neutrophil-platelet aggregates are dynamically formed in the lung in response to injury and are regulated by aspirin-triggered lipoxin.
The therapeutic effect of aspirin in acute lung injury is in large part mediated by the production of pro-resolving lipid mediators.
Abstract
Evidence is emerging that platelets are major contributors to innate immune responses in conditions such as acute lung injury (ALI). Platelets form heterotypic aggregates with neutrophils, and we hypothesized that lipoxin mediators regulate formation of neutrophil-platelet aggregates (NPA) and that NPA significantly contribute to ALI. Lipopolysaccharide (LPS)-induced lung injury was accompanied by platelet sequestration, activation, intra-alveolar accumulation, and NPA formation within both blood and alveolar compartments. Using lung intravital microscopy, we observed the dynamic formation of NPA during physiologic conditions, which sharply increased with ALI. Aspirin (ASA) treatment significantly reduced lung platelet sequestration and activation, NPA formation, and lung injury. ASA treatment increased levels of ASA-triggered lipoxin (ATL; 15-epi-lipoxin A4), and blocking the lipoxin A4 receptor (ALX) with a peptide antagonist (Boc2) or using ALX knockouts (Fpr2/3−/−) reversed this protection. LPS increased NPA formation in vitro, which was reduced by ATL, and engagement of ALX by ATL on both neutrophils and platelets was necessary to prevent aggregation. In a model of transfusion-related acute lung injury (TRALI), Boc2 also reversed ASA protection, and treatment with ATL in both LPS and TRALI models protected from ALI. We conclude that ATL regulates neutrophil-platelet aggregation and that platelet-neutrophil interactions are a therapeutic target in lung injury.
Introduction
The acute respiratory distress syndrome (ARDS) is a common and often fatal syndrome characterized by neutrophilic inflammation. In ARDS and experimental models of acute lung injury (ALI), an exuberant inflammatory response with release of proteases and other toxic mediators from activated leukocytes leads to tissue damage, increased permeability of the alveolar-capillary barrier, and the formation of protein-rich lung edema.1
Several lines of evidence indicate that activated platelets act as important effectors in host defense, influencing pulmonary neutrophil recruitment and contributing to the development of ALI.2-4 Platelets become activated within the blood circulation at sites of inflammation and can adhere to other platelets, to leukocytes, forming neutrophil-platelet aggregates (NPAs) or monocyte-platelet aggregates (MPAs), and to exposed endothelium.5 The presence of circulating leukocyte-platelet aggregates (LPAs) is a sensitive indicator of platelet activation and has been observed in patients with inflammatory conditions including sepsis, coronary diseases, rheumatoid arthritis, inflammatory bowel disease, cystic fibrosis, and ARDS.6-10
Activated platelets synthesize thromboxane A2 from arachidonic acid via a sequential reaction catalyzed by cyclooxygenase-2 (COX-2). Aspirin (acetylsalicylic acid [ASA]) acetylates COX-2, and this conformational change leads to inhibition of prostanoid synthesis.11 Acetylation of COX-2 switches catalytic activity to convert arachidonic acid to 15R-hydroxyeicosatetraenoic acid, which can be subsequently converted to 15(R)-epi-lipoxin A4 (15[R]-epi-LXA4), also known as ASA-triggered lipoxin (ATL).12 Lipoxins are endogenous lipid mediators generated during inflammation that can block inflammatory cell recruitment, inhibit cytokine release, and decrease vascular permeability, which collectively are anti-inflammatory properties.13,14 Lipoxins also stimulate epithelial reconstitution, facilitate apoptosis, and promote macrophage efferocytosis, which collectively are proresolution properties.13,14 Production of lipoxins requires transcellular biosynthesis, with leukocytes, platelets, and tissue resident cells having important roles.12,15
ATL shares the potent anti-inflammatory actions of lipoxins but is more resistant to metabolic inactivation.16 The anti-inflammatory, proresolving properties of both LXA4 and 15(R)-epi-LXA4 are mediated through the formyl peptide receptor type 2 (termed Fpr2/3 in the mouse), also called the lipoxin A4 receptor (ALX),17 which is expressed in a variety of cell types including endothelial cells and myeloid lineages.17,18 In addition to native lipoxins and ATL, ALX recognizes a diverse array of bioactive mediators including annexin A1, resolvin D1, and serum amyloid A protein.17,19 Recent work has elucidated the molecular mechanisms behind the dual actions of ALX (pro- vs anti-inflammatory responses), which is determined by unique receptor dimerization patterns induced by specific ligands.20
Here, we demonstrate the role of ATL in regulating leukocyte-platelet aggregation, and consequently lung injury, in both direct and indirect experimental models of ALI: intratracheal instillation of lipopolysaccharide (LPS) and a 2-event model of transfusion-related acute lung injury (TRALI).21 We present evidence that platelets have a central role in producing pulmonary inflammation and injury based on critical interactions with neutrophils that are regulated by ATL.
Methods
Reagents
The following reagents were used: ASA, LPS from Escherichia coli O55:B5 (Sigma-Aldrich), Boc2 (Genway), and 15-epi-LXA4 (EMD Biosciences).
Intratracheal LPS model
Experimental procedures were performed in 8- to 12-week-old male BALB/c wild-type (WT) mice (Charles River) housed under pathogen-free conditions in the Animal Barrier Facility at University of California, San Francisco. Mice were pretreated (intraperitoneally) with 0.1 mg/g of ASA or dimethylsulfoxide (DMSO) vehicle, 24 and 2 hours before ALI induction. Mice were challenged with intratracheal phosphate-buffered saline (PBS; controls) or LPS (5 μg/g), and analysis was performed 4 to 48 hours later. In selected experiments, mice received 10 μg/kg intraperitoneally of Boc2 30 minutes before ASA injections and 24 hours after LPS instillation or 100 to 5000 ng/mouse of 15-epi-LXA4 30 minutes before and 24 hours after LPS challenge. There is no appropriate peptide control for Boc2. Fpr2/3−/− mice were obtained from Rod Flower (St. Barts and the London School of Medicine and Dentistry).19
Bronchoalveolar lavage (BAL) was obtained at 48 hours by placing a catheter into the trachea, through which 1 mL of cold PBS was flushed back three times. Protein concentration in the cell-free BAL was determined using a BCA protein assay kit (Thermo Scientific). BAL leukocytes were counted using a Coulter counter (Beckman Coulter), and differential was determined by cytospin preparation (Cytospin 3; Thermo Electron Corp.) and Diff-Quick staining. BAL hemoglobin was measured using LowHb microcuvettes (HemoCue).
TRALI model
A 2-event TRALI model was used, as previously described.21 Briefly, after being primed with LPS (0.1 mg/kg, intraperitoneally) for 24 hours, mice were challenged with MHC I monoclonal antibodies (mAb) (H2Kd; immunoglobulin G2a, κ; 1.0 mg/kg) injected into the jugular vein and euthanized after 2 hours. To quantify pulmonary edema formation, bloodless, extravascular lung water was measured.21,22 We also measured lung vascular permeability to protein by instilling mice with intravenous 125I-labeled albumin. The radioactivity in the blood and the bloodless lung was measured with a gamma counter (Packard 5000 Series), and the ratio was used to calculate the lung extravascular plasma equivalents (EVPEs).21,22 ASA (0.1 mg/g), or DMSO control, was delivered intraperitoneally 30 minutes prior to LPS priming and again 2 hours prior to H2Kd mAb challenge. Boc2 (10 μg/kg), or vehicle (PBS), was administrated intraperitoneally 30 minutes prior to the ASA injections. 15-Epi-LXA4, or vehicle (ethanol), was administered via tail vein 30 minutes prior to H2Kd mAb injections.
Immunohistochemistry
For histological analysis, 7 μm of optimal cutting temperature compound-embedded frozen lung sections (without lung perfusion) was fixed in cold acetone and blocked with PBS containing 8% rabbit serum and 4% bovine serum albumin. Sections were incubated with CD41 antibody (clone MWReg30; BD Biosciences), followed by peroxidase-conjugated secondary Abs, color development, and hematoxylin counterstaining.21
Western blot
Western blots on digested lungs and BAL samples were done using standard techniques and immunoblotted with CD41 and α-tubulin Abs.
Enzyme-linked immunosorbent assay
15-epi-LXA4 and thromboxane B2 concentrations in plasma and BAL were determined using enzyme-linked immunosorbent assay kits from Neogen and GE Healthcare, respectively. We followed the manufacturer’s instructions for lipid extraction.
MPO activity
Cell-free BAL and myeloperoxidase (MPO) standards (Sigma-Aldrich) were placed onto a 96-well plate, and 200 μL of o-dianisidine solution and 10 μL of 0.1% H2O2 were added. The absorbance was read after 5 minutes at 405 nm and expressed as units per milliliter of BAL supernatant using a standard curve.21
Flow cytometry
Whole blood was collected into acid citrate dextrose (Sigma-Aldrich). Samples in the presence of FcγRII/III blocking Ab (2.4G2) were diluted with Tyrode’s buffer supplemented with 10 U/mL of heparin (APP), 7 U/mL Apyrase (Sigma-Aldrich), and 0.5 μM prostaglandin I2 (Sigma-Aldrich). eFluor450-CD11b (eBioscience), phycoerythrin-Ly6G (BD Bioscience), allophycocyanin-CD41 (eBioscience), and isotope controls Abs were used to detect leukocyte and platelets antigens. Samples were examined with a LSRII/Fortessa flow cytometer (BD Bioscience). Neutrophils and monocytes were gated by their forward- and side-scatter characteristics and by their Ly-6G+/CD11b+ (neutrophil) or Ly-6G−/CD11b+/ Ly-6C+ (monocyte) expression pattern. Platelet-neutrophil or platelet-monocyte aggregates were detected by CD41 Ab staining. In selected experiments, whole lungs were digested,21 followed by the same general protocol described above. All data were analyzed using FlowJo software (TreeStar).
In vitro neutrophil-platelet aggregate experiments
Whole blood was collected from C57BL/6 WT and Fpr2/3−/− mice into acid citrate dextrose and used to isolate platelets.23 Bone marrow neutrophils were isolated, as previously described.24 Neutrophils and platelets (1:100 ratio) from WT and Fpr2/3−/− mice were incubated for 1h at 37°C with LPS (5 μg/mL) or with 15-epi-LXA4 (300 nM), in presence of Tyrode’s buffer. After this incubation, Tyrode’s buffer with supplements (see above) was added. Flow cytometry was performed as described above.
Lung intravital microscopy
We used 2-photon intravital microscopy25 to record in real-time neutrophil and platelet interactions in LPS-induced ALI. To facilitate tracking of both platelets and neutrophils, PF4-cre × Rosa26-LSL-tdTomato mice (both from The Jackson Laboratories) were crossed with LysM-eGFP mice (obtained from E. Robey, University of California, Berkeley, CA). To permit identification of the lung vasculature, Cascade-blue dextran (50 μL of 25 mg/mL; Life Technologies) was injected into the jugular vein. Mice were treated with LPS ± ASA and compared with LPS untreated mice (PBS). We captured a 0.4-mm2 x-y surface area at 40 μm z-depth, capturing a complete image every 1 minute for 60 minutes. Images were analyzed using Imaris 7.6.1 software (Bitplane) to quantify mobile neutrophils (green) that colabeled for platelets (red). The number of events was averaged per minute, and the mean of the highest 20 values obtained was recorded as the number of neutrophil-platelet aggregates per 0.1 mm2 of lung tissue at 40 μm z-depth.
Statistics
All in vivo and in vitro experiments were repeated a minimum of 3 independent times. Results are reported as both individual data points and mean ± standard deviation (SD). To determine significance, 2-tailed Student t test, analysis of variance, and log-rank (Mantel-Cox) tests were used as appropriate (GraphPad PRISM version 5.0). P ≤ .05 was deemed to be significant.
Study approval
All experiments were approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco.
Results
Intratracheal LPS induces platelet sequestration and activation within the lungs and ASA attenuates these responses
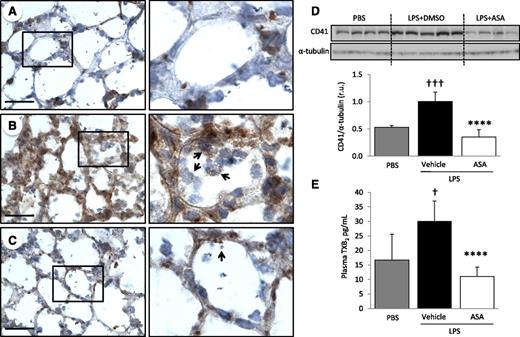
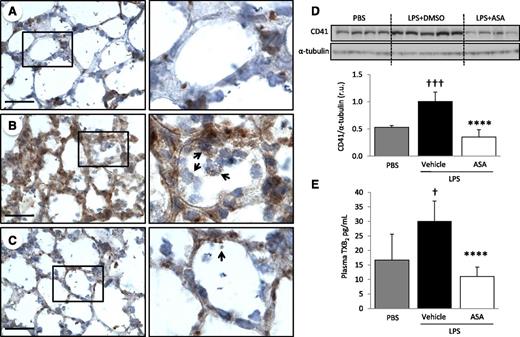
To determine the role of platelets in experimental ALI, we used an intratracheal LPS model of lung injury. Immunohistochemistry for the platelet specific marker CD41, 48 hours after intratracheal LPS instillation (5 µg/g), revealed intense sequestration of platelets within LPS-injured lungs compared with PBS-instilled control animals (Figure 1A-B). We cannot rule out that areas of particularly intense CD41 staining at vessel junctions could indicate the presence of megakaryocytes. CD41 protein expression in total lung digest was analyzed by western blot and confirmed the sequestration of platelets within the lungs of LPS-challenged animals (Figure 1D). Thromboxane A2 is a marker of platelet activation and is rapidly hydrolyzed to its inactive stable metabolite, thromboxane B2 (TXB2). Plasma TXB2 levels were increased after LPS challenge (Figure 1E). Pretreatment with ASA 24 and 2 hours before LPS challenge prevented platelet accumulation in the lungs (Figure 1C-D) and decreased plasma TXB2 levels (Figure 1E) compared with controls (DMSO).
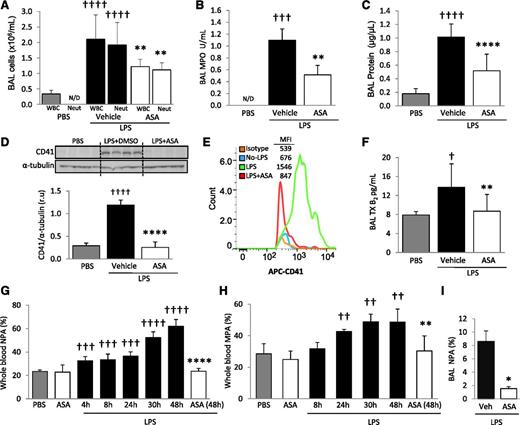
Platelets are sequestered and activated in the lungs after intratracheal LPS and ASA attenuates these responses. CD41 immunostaining of lung tissue sections from (A) control mice (PBS), (B) LPS-challenged mice treated with vehicle (DMSO), or (C) LPS-challenged mice treated with ASA. Images are representative of ≥3 animals per group. Original magnification, ×60; zoom of outlined areas. Scale bar = 20 µm. Arrows indicate CD41+ events in the intra-alveolar spaces. (D) CD41 protein expression and densitometric analysis from lung digestion samples in PBS controls and LPS-challenged mice ± ASA. †††P < .001 vs PBS; ****P < .0001 vs vehicle. (E) Platelet activation was assessed by measuring TXB2 plasma levels in PBS and LPS-challenged mice ± ASA. †P < .05 vs PBS; ****P < .0001 vs vehicle. Data are mean ± SD of 4 to 9 animals per group.
Platelets are sequestered and activated in the lungs after intratracheal LPS and ASA attenuates these responses. CD41 immunostaining of lung tissue sections from (A) control mice (PBS), (B) LPS-challenged mice treated with vehicle (DMSO), or (C) LPS-challenged mice treated with ASA. Images are representative of ≥3 animals per group. Original magnification, ×60; zoom of outlined areas. Scale bar = 20 µm. Arrows indicate CD41+ events in the intra-alveolar spaces. (D) CD41 protein expression and densitometric analysis from lung digestion samples in PBS controls and LPS-challenged mice ± ASA. †††P < .001 vs PBS; ****P < .0001 vs vehicle. (E) Platelet activation was assessed by measuring TXB2 plasma levels in PBS and LPS-challenged mice ± ASA. †P < .05 vs PBS; ****P < .0001 vs vehicle. Data are mean ± SD of 4 to 9 animals per group.
ASA decreases LPS-induced lung injury and alveolar platelet accumulation
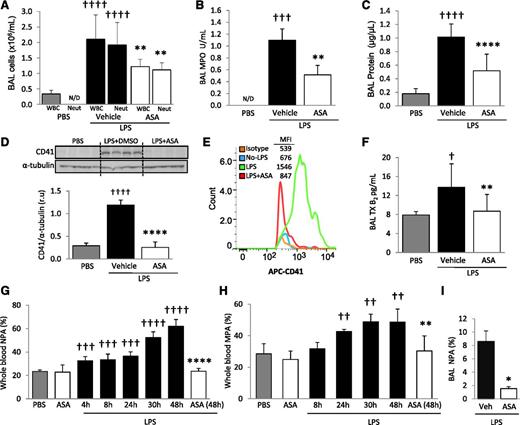
The total number of white blood cells (WBCs) and neutrophils in BAL was measured 48 hours after intratracheal PBS (control) or LPS instillation. MPO activity, an index of neutrophil activation, was also measured in cell-free BAL supernatant. The number of WBCs and neutrophils within BAL increased with LPS challenge, as did MPO activity (Figure 2A-B). BAL total protein was measured as an indicator of lung permeability and increased after LPS instillation (Figure 2C). Mice treated with ASA had significantly decreased BAL WBCs, neutrophils, MPO activity, and total protein compared with vehicle controls (Figure 2A-C).
LPS produces ALI, increased blood NPA and MPA, and the intra-alveolar accumulation of platelets and NPA; ASA attenuates these responses. (A) BAL WBC and neutrophil counts, (B) MPO activity in the cell-free BAL, and (C) lung protein permeability in PBS and LPS-challenged mice + ASA vs vehicle (DMSO) controls. **P < .01, ****P < .0001 vs vehicle groups and †††P < .001, ††††P < .0001 vs PBS. (D) Densitometric analysis and representative membrane of blots against CD41 protein performed on BAL cells in PBS controls and LPS + ASA vs vehicle group. ††††P < .0001 and ****P < .0001 vs PBS and vehicle, respectively. (E) CD41 (and isotype control) flow cytometry analysis in BAL cells from mice with no LPS and LPS ± ASA with representative histogram plots and MFI (representative of 5 independent experiments). (F) TXB2 levels in cell-free BAL from control and LPS + ASA vs vehicle groups. †P < .05 vs PBS; **P < .05 vs vehicle. (G) Percentage NPAs and (H) percentage MPAs in whole blood at different time points after LPS instillation and effects of ASA treatment at indicated times. ††P < .01, †††P < .001, and ††††P < .0001 vs PBS; and **P < .01 and ****P < .0001 vs LPS-challenged mice at 48 hours. (I) Percentage NPAs in BAL after LPS + ASA vs vehicle (at 48 hours). *P < .05 vs vehicle. Data are mean ± SD of 5 to 10 animals per group.
LPS produces ALI, increased blood NPA and MPA, and the intra-alveolar accumulation of platelets and NPA; ASA attenuates these responses. (A) BAL WBC and neutrophil counts, (B) MPO activity in the cell-free BAL, and (C) lung protein permeability in PBS and LPS-challenged mice + ASA vs vehicle (DMSO) controls. **P < .01, ****P < .0001 vs vehicle groups and †††P < .001, ††††P < .0001 vs PBS. (D) Densitometric analysis and representative membrane of blots against CD41 protein performed on BAL cells in PBS controls and LPS + ASA vs vehicle group. ††††P < .0001 and ****P < .0001 vs PBS and vehicle, respectively. (E) CD41 (and isotype control) flow cytometry analysis in BAL cells from mice with no LPS and LPS ± ASA with representative histogram plots and MFI (representative of 5 independent experiments). (F) TXB2 levels in cell-free BAL from control and LPS + ASA vs vehicle groups. †P < .05 vs PBS; **P < .05 vs vehicle. (G) Percentage NPAs and (H) percentage MPAs in whole blood at different time points after LPS instillation and effects of ASA treatment at indicated times. ††P < .01, †††P < .001, and ††††P < .0001 vs PBS; and **P < .01 and ****P < .0001 vs LPS-challenged mice at 48 hours. (I) Percentage NPAs in BAL after LPS + ASA vs vehicle (at 48 hours). *P < .05 vs vehicle. Data are mean ± SD of 5 to 10 animals per group.
CD41 immunohistochemistry of lungs after LPS challenge revealed intense sequestration of platelets within the pulmonary capillaries but also suggested the presence of intra-alveolar platelets (Figure 1B, inset). To further test for intra-alveolar platelet accumulation, we probed for CD41 protein via western blot analysis on cells obtained from BAL. CD41 protein was increased in BAL from LPS-challenged mice compared with PBS controls (Figure 2D). We also used flow cytometry and a CD41 mAb to determine the presence of platelets in the BAL and did not detect intra-alveolar platelets in control animals, but a sharp increase was detected after LPS challenge (Figure 2E). We tested for pulmonary hemorrhage as a potential cause of intra-alveolar accumulation of platelets by measuring BAL hemoglobin and found a minimal increase in hemoglobin in the LPS-challenged animals (supplemental Figure 1, available on the Blood Web site). We detected significantly increased TXB2 concentrations in the cell-free BAL supernatant of LPS-treated mice compared with controls (Figure 2F). These results demonstrate that platelets accumulate and are activated in the alveolar spaces after LPS challenge. ASA treatment yielded a significant reduction in both alveolar LPS-induced CD41 expression (Figure 2D-E) and platelet activation (Figure 2F) and did not affect BAL hemoglobin measurements.
Leukocyte-platelet aggregates are present in the blood and alveolar spaces after LPS-challenge and are reduced with ASA treatment
We investigated the LPS-induced formation of LPAs in whole blood and BAL using flow cytometry. The gating strategies for whole blood and BAL are illustrated in supplemental Figure 2A-B. Briefly, antibodies against the leukocyte-specific surface markers CD11b, Ly6G, or Ly6C, in addition to the platelet-specific marker CD41, were used to identify MPAs, defined as CD11b+/Ly6C+/Ly6G−/CD41+, and NPAs, defined as CD11b+/Ly6G+/CD41+. Whole blood obtained by cardiac puncture revealed that LPS induced both NPA (Figure 2G) and MPA (Figure 2H) formation as early as 4 and 24 hours, respectively, after LPS instillation. Blood NPA and MPA levels progressively increased over time, reaching a maximum at 48 hours after LPS instillation (NPA = 62 ± 5.6%, MPA = 48.6 ± 3.4%). The percentage of NPA in the BAL of LPS-challenged mice also increased (Figure 2I), but we did not detect a similar increase in BAL MPAs (data not shown). Treatment with ASA significantly decreased blood NPA and MPA formation, as well as BAL NPA formation, at 48 hours after LPS (Figure 2G-I).
Dynamic formation of NPAs within the lung microcirculation
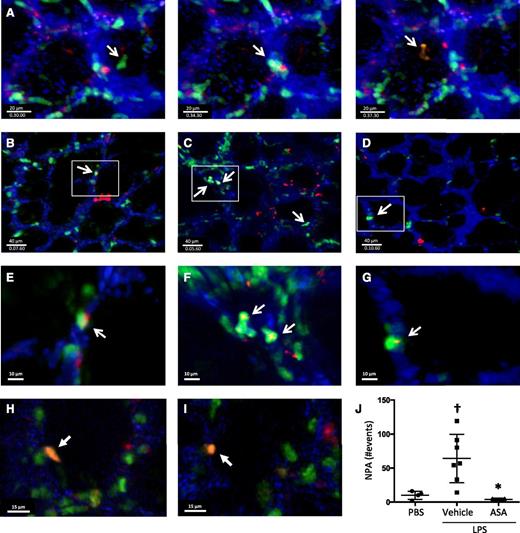
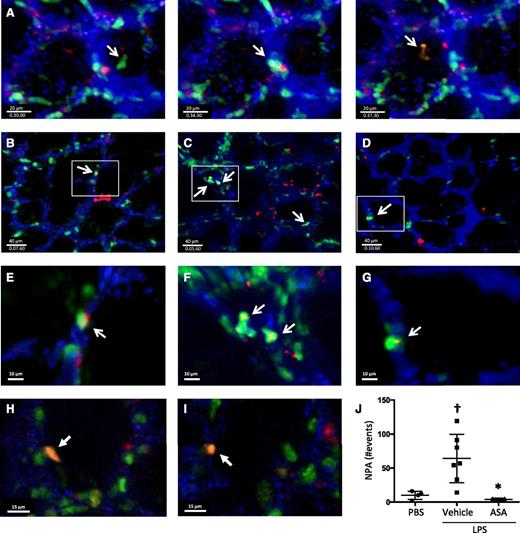
We used 2-photon intravital lung microscopy25 to determine the spatial and temporal formation of NPA within the pulmonary microcirculation at baseline and after LPS-induced ALI. To track platelets, Rosa26-LSL-tdTomato mice were crossed with PF4-cre mice,26 and in the peripheral blood of these mice, >99% of the tdTomato+ events were also CD41+ (supplemental Figure 3). These mice were crossed with LysM-eGFP mice to facilitate neutrophil identification. Cascade-blue dextran was injected intravenously to delineate the pulmonary microvasculature. In the triple transgenic mice without LPS exposure, we observed the dynamic formation of NPA within the lung microcirculation, with intravascular neutrophils interacting with platelets (example shown in Figure 3A and supplemental Movie 1). We also observed NPAs in PBS-challenged mice (Figure 3B,E; supplemental Movie 2), and in the first hours after LPS exposure, there was a sharp increase in NPA formation (Figure 3C,F; supplemental Movies 3 and 4), which was ameliorated with ASA pretreatment, with NPA formation decreasing to basal levels (Figure 3D,G; supplemental Movie 5). At later time points after LPS challenge (24 hours), we detected immobile NPAs that were outside of the intravascular dextran signal (Figure 3H-I; supplemental Movie 9), consistent with intra-alveolar NPAs. Areas of neutrophil-platelet colocalization were analyzed by surface rendering (supplemental Movies 6-8) and quantified in Figure 3J.
Lung intravital microscopy reveals the dynamic formation of NPAs in the lungs under homeostatic and injury conditions. (A) Screenshots of a PF4-cre × Rosa26-LSL-tdTomato × LysM-eGFP mouse under basal conditions showing the dynamic formation of NPAs in the lung microcirculation (supplemental Movie 1). (B-G) Screenshots with zoom insets of PF4-cre × Rosa26-LSL-tdTomato × LysM-eGFP mice challenged intratracheally with (B,E) PBS, (C,F) LPS + vehicle, or (D,G) LPS + ASA and imaged directly after challenge (supplemental Movies 2-8). White arrows indicate NPAs. (H-I) Screenshots from PF4-cre × Rosa26-LSL-tdTomato × LysM-eGFP mice challenged with intratracheally LPS and imaged 24 hours after instillation. White arrows indicate intra-alveolar NPA (supplemental Movie 9). (J) Quantification of NPAs in B-D. †P < .05 vs PBS; *P < .05 vs vehicle. Data are mean ± SD of 3 to 7 animals per group.
Lung intravital microscopy reveals the dynamic formation of NPAs in the lungs under homeostatic and injury conditions. (A) Screenshots of a PF4-cre × Rosa26-LSL-tdTomato × LysM-eGFP mouse under basal conditions showing the dynamic formation of NPAs in the lung microcirculation (supplemental Movie 1). (B-G) Screenshots with zoom insets of PF4-cre × Rosa26-LSL-tdTomato × LysM-eGFP mice challenged intratracheally with (B,E) PBS, (C,F) LPS + vehicle, or (D,G) LPS + ASA and imaged directly after challenge (supplemental Movies 2-8). White arrows indicate NPAs. (H-I) Screenshots from PF4-cre × Rosa26-LSL-tdTomato × LysM-eGFP mice challenged with intratracheally LPS and imaged 24 hours after instillation. White arrows indicate intra-alveolar NPA (supplemental Movie 9). (J) Quantification of NPAs in B-D. †P < .05 vs PBS; *P < .05 vs vehicle. Data are mean ± SD of 3 to 7 animals per group.
ASA treatment increases plasma and BAL 15-epi-LXA4 concentrations, and blockade of ALX reverses the protective effects of ASA
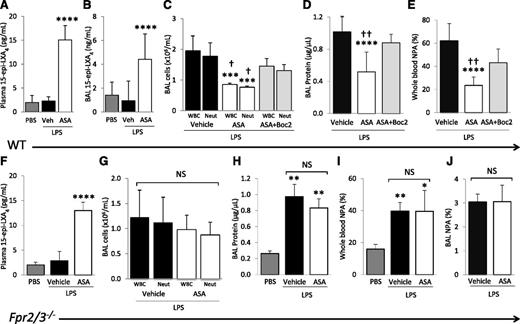
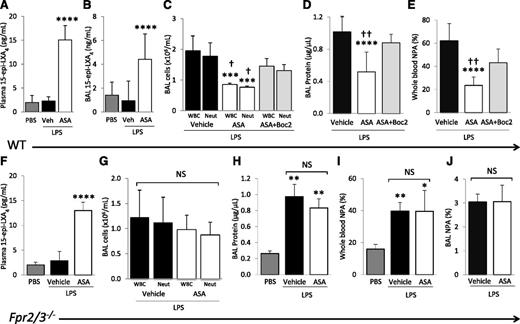
Plasma and BAL 15-epi-LXA4 concentrations were measured by enzyme-linked immunosorbent assay after PBS and LPS challenge ± aspirin treatment. LPS did not affect plasma or BAL 15-epi-LXA4 levels, but ASA treatment significantly increased both plasma and BAL 15-epi-LXA4 concentrations in LPS-challenged mice at 48 hours compared with PBS or vehicle controls (Figure 4A-B).
Blocking the lipoxin receptor reverses ASA protective effects. (A) 15-Epi-LXA4 levels in plasma and (B) cell-free BAL of WT mice challenged with PBS or LPS + ASA vs vehicle (DMSO). ****P < .001 vs PBS and vehicle groups. (C) BAL WBC and neutrophil counts, (D) BAL cell-free total protein, and (E) whole blood NPAs in WT mice challenged with LPS + ASA vs vehicle or ASA + Boc2. ***P < .001 and ****P < .0001 vs vehicle groups, respectively. †P < .05 and ††P < .01 vs ASA + Boc2 groups, respectively. (F) 15-Epi-LXA4 levels in plasma of Fpr2/3−/− mice challenged with PBS or LPS + ASA vs vehicle (DMSO). ****P < .0001 vs PBS and vehicle groups. (G) BAL WBC and neutrophil counts, (H) BAL cell-free total protein, (I) NPAs in whole blood, and (J) NPAs in BAL cells of Fpr2/3−/− mice challenged with PBS or LPS + ASA vs vehicle. *P < .05, **P < .01 vs PBS. NS, not significant. Data are mean ± SD of 5 to 7 animals per group.
Blocking the lipoxin receptor reverses ASA protective effects. (A) 15-Epi-LXA4 levels in plasma and (B) cell-free BAL of WT mice challenged with PBS or LPS + ASA vs vehicle (DMSO). ****P < .001 vs PBS and vehicle groups. (C) BAL WBC and neutrophil counts, (D) BAL cell-free total protein, and (E) whole blood NPAs in WT mice challenged with LPS + ASA vs vehicle or ASA + Boc2. ***P < .001 and ****P < .0001 vs vehicle groups, respectively. †P < .05 and ††P < .01 vs ASA + Boc2 groups, respectively. (F) 15-Epi-LXA4 levels in plasma of Fpr2/3−/− mice challenged with PBS or LPS + ASA vs vehicle (DMSO). ****P < .0001 vs PBS and vehicle groups. (G) BAL WBC and neutrophil counts, (H) BAL cell-free total protein, (I) NPAs in whole blood, and (J) NPAs in BAL cells of Fpr2/3−/− mice challenged with PBS or LPS + ASA vs vehicle. *P < .05, **P < .01 vs PBS. NS, not significant. Data are mean ± SD of 5 to 7 animals per group.
To determine whether elevated 15-epi-LXA4 contributed to the decreased lung injury observed after aspirin treatment, we tested the ALX (Fpr2/3) antagonist, Boc2.27 The administration of Boc2 30 minutes before aspirin treatment, and repeated 24 hours after LPS instillation, reversed the protective effects of aspirin on lung injury (Figure 4C-D). Additionally, Boc2 partially reversed the decrease in whole blood NPAs after ASA treatment (Figure 4E). Boc2 treatment alone (in the absence of ASA) had no effect on LPS-induced lung inflammation or injury (data not shown). Also, Boc2 did not change the plasma 15-epi-LXA4 concentrations of ASA treated mice (data not shown).
Next, we confirmed the results obtained with pharmacologic blockade of Fpr2/3 using the recently characterized Fpr2/3−/− mice.19 ASA treatment of Fpr2/3−/− animals increased plasma concentrations of 15-epi-LXA4 in the LPS model (Figure 4F). Compared with WT controls, Fpr2/3−/− mice had no significant differences in BAL WBCs, neutrophils, or total protein after LPS challenge (data not shown), and we observed no significant decreases in BAL WBCs, neutrophils, or total protein after ASA treatment (Figure 4G-H). The ASA-induced decrease in blood and BAL NPA was also blocked in Fpr2/3−/− mice (Figure 4I-J).
Effects of 15-epi-LXA4 on LPS-induced lung injury
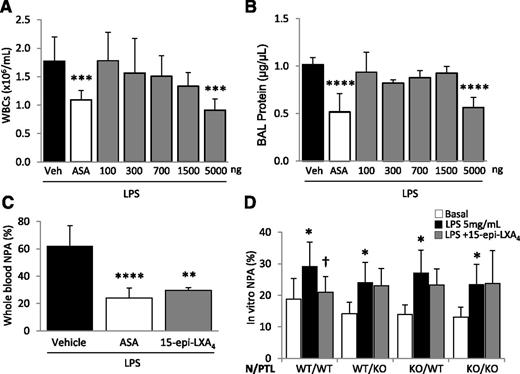
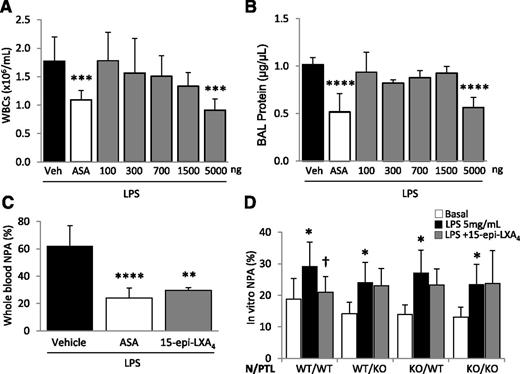
To evaluate whether pharmacologic doses of 15-epi-LXA4 can modulate LPS-induced lung injury, mice were treated with increasing doses of this lipid mediator. Intravenous administration of between 100 and 1500 ng of 15-epi-LXA4 did not produce a significant change in BAL WBC counts or total protein after LPS challenge (Figure 5A-B). However, doses of 5 µg resulted in a significant decrease in BAL WBCs (Figure 5A) and attenuated lung permeability (Figure 5B) and whole blood NPA formation (Figure 5C) in a similar manner to that observed with aspirin treatment.
Treatment with 15-epi-LXA4 decreases LPS-induced ALI and regulates NPA formation in vivo and in vitro. (A-B) ASA or 15-epi-LXA4 treatment (at doses indicated) 2 hours before and 24 hours after LPS challenge and (A) leukocyte recruitment into the air spaces and (B) BAL total protein. ***P < .001, ****P < .0001 vs vehicle groups. (C) Whole blood NPAs in mice challenged with LPS ± ASA or 15-epi-LXA4 (5 µg). **P < .01 and ****P < .0001 vs vehicle. (D) Neutrophils and platelets isolated from WT or Fpr2/3−/− mice were incubated with LPS (5 µg/mL) for 1 hour to produce NPAs in vitro. LPS induced NPA in all combinations of WT and Fpr2/3−/− neutrophils/platelets. *P < .05 vs basal (no LPS). Pretreatment with 15-epi-LXA4 reduced NPA formation after LPS in only the WT neutrophil/WT platelet group. †P < .05. Data are (A-C) mean ± SD of 5 animals per group or (D) mean ± SD of ≥3 independent experiments.
Treatment with 15-epi-LXA4 decreases LPS-induced ALI and regulates NPA formation in vivo and in vitro. (A-B) ASA or 15-epi-LXA4 treatment (at doses indicated) 2 hours before and 24 hours after LPS challenge and (A) leukocyte recruitment into the air spaces and (B) BAL total protein. ***P < .001, ****P < .0001 vs vehicle groups. (C) Whole blood NPAs in mice challenged with LPS ± ASA or 15-epi-LXA4 (5 µg). **P < .01 and ****P < .0001 vs vehicle. (D) Neutrophils and platelets isolated from WT or Fpr2/3−/− mice were incubated with LPS (5 µg/mL) for 1 hour to produce NPAs in vitro. LPS induced NPA in all combinations of WT and Fpr2/3−/− neutrophils/platelets. *P < .05 vs basal (no LPS). Pretreatment with 15-epi-LXA4 reduced NPA formation after LPS in only the WT neutrophil/WT platelet group. †P < .05. Data are (A-C) mean ± SD of 5 animals per group or (D) mean ± SD of ≥3 independent experiments.
To determine whether lipoxin receptor signaling on neutrophils and/or platelets mediated the protective effects observed after aspirin and 15-epi-LXA4 treatment, we developed a coculture system in which neutrophils and platelets, freshly isolated from WT or Fpr2/3−/− mice, were incubated with LPS (5 μg/mL) to induce NPA formation. We observed a basal formation of NPA after a 1-hour incubation of WT neutrophils and platelets (1:100), which increased after LPS treatment (Figure 5D). Pretreatment with 15-epi-LXA4 (300 nM) significantly decreased LPS-induced NPA formation (Figure 5D). We next tested combinations of WT and Fpr2/3−/− neutrophils and platelets and observed that the presence of a functional lipoxin receptor was required on both neutrophils and platelets for 15-epi-LXA4 to decrease NPA formation (Figure 5D).
Lipoxins enhance inflammatory resolution in TRALI
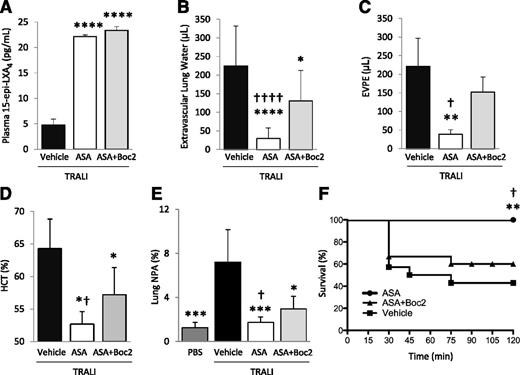
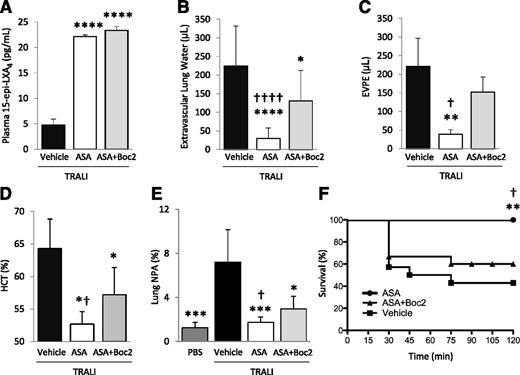
We previously demonstrated that ASA treatment decreases platelet sequestration, lung vascular permeability, and edema formation, and increases survival in an experimental TRALI model.2,24 Here, we investigated whether ATL was required for these effects. In a 2-event model of TRALI,21 we treated animals with aspirin ± Boc2, or vehicle (DMSO), 30 minutes prior to the intraperitoneal LPS priming, and again 2 hours prior to challenge with H2Kd mAb. Both aspirin and ASA + Boc2 treatment significantly increased 15-epi-LXA4 concentrations in this model (Figure 6A). Lung edema formation, lung vascular permeability, hemoconcentration, and mortality were all reduced with ASA treatment compared with vehicle-treated controls (Figure 6B-D,F). Extravascular lung water and EVPE values in LPS-primed controls are 0 and <10 µL, respectively.21 Hematocrit values in LPS-primed controls are ∼50%.21 TRALI induced the formation of lung NPA, which was ameliorated by aspirin treatment (Figure 6E). Pretreatment with Boc2 significantly reversed the protective effects of aspirin on lung injury, NPA formation, and mortality (Figure 6B-F). The administration of Boc2 alone did not affect TRALI severity (data not shown).
Boc2 blocks the protective effects of ASA in a 2-event model of TRALI. BALB/c WT mice were treated with LPS (0.1 mg/kg, intraperitoneally) and 24 hours later were challenged with H2Kd mAb (1.0 mg/kg, intravenously) and euthanized 2 hours later. (A) Plasma 15-epi-LXA4 levels in mice with TRALI + vehicle, ASA, or ASA + Boc2. ****P < .0001 vs vehicle group. (B) Extravascular lung water, (C) EVPE, and (D) hematocrit in mice with TRALI + vehicle, ASA, or ASA + Boc2. *P < .05, **P < .01, and ****P < .0001 vs vehicle groups. †P < .05 and ††††P < .0001 vs ASA + Boc2 groups. (E) NPAs in the lung digestion in mice with TRALI + vehicle, ASA, or ASA + Boc2. *P < .05 and ***P < .001 vs vehicle group. †P < .05 vs ASA + Boc2 group. (F) Survival curves in mice with TRALI + vehicle, ASA, or ASA + Boc2. **P < .01 vs vehicle group and †P < .05 vs ASA + Boc2 group. Data are mean ± SD of 5 to 10 animals per group.
Boc2 blocks the protective effects of ASA in a 2-event model of TRALI. BALB/c WT mice were treated with LPS (0.1 mg/kg, intraperitoneally) and 24 hours later were challenged with H2Kd mAb (1.0 mg/kg, intravenously) and euthanized 2 hours later. (A) Plasma 15-epi-LXA4 levels in mice with TRALI + vehicle, ASA, or ASA + Boc2. ****P < .0001 vs vehicle group. (B) Extravascular lung water, (C) EVPE, and (D) hematocrit in mice with TRALI + vehicle, ASA, or ASA + Boc2. *P < .05, **P < .01, and ****P < .0001 vs vehicle groups. †P < .05 and ††††P < .0001 vs ASA + Boc2 groups. (E) NPAs in the lung digestion in mice with TRALI + vehicle, ASA, or ASA + Boc2. *P < .05 and ***P < .001 vs vehicle group. †P < .05 vs ASA + Boc2 group. (F) Survival curves in mice with TRALI + vehicle, ASA, or ASA + Boc2. **P < .01 vs vehicle group and †P < .05 vs ASA + Boc2 group. Data are mean ± SD of 5 to 10 animals per group.
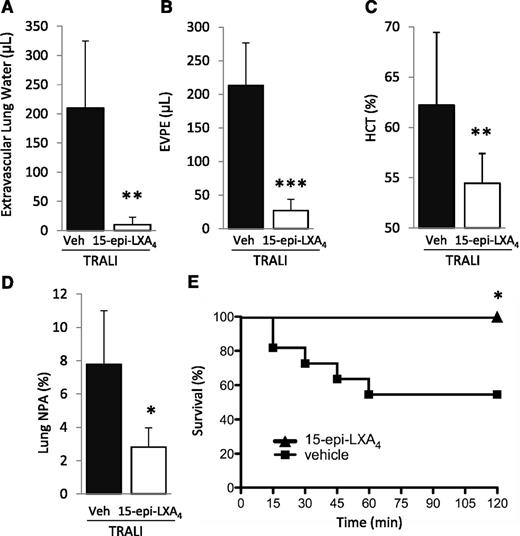
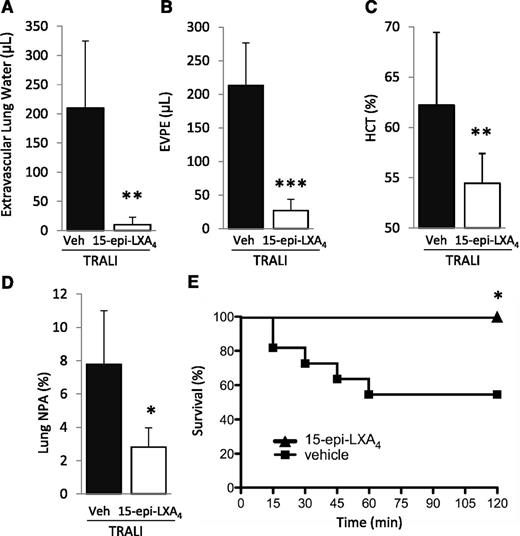
We subsequently tested the impact of treatment with 15-epi-LXA4 or vehicle (intravenous) administered 30 minutes prior to H2Kd mAb challenge on lung injury and NPA formation. 15-Epi-LXA4 treatment significantly decreased lung injury and formation of lung NPA and eliminated mortality compared with vehicle-treated animals (Figure 7A-E).
15-Epi-LXA4 protects mice from TRALI. (A) Extravascular lung water, (B) EVPE, (C) NPAs in the lung digestion and (D) hematocrit in WT mice with TRALI + 15-epi-LXA4 vs vehicle. *P < .05, **P < .01, and ***P < .001 vs vehicle groups. (E) Survival curves in mice with TRALI +15-epi-LXA4 vs vehicle. *P < .05 vs vehicle group. Data are mean ± SD of 10 animals per group.
15-Epi-LXA4 protects mice from TRALI. (A) Extravascular lung water, (B) EVPE, (C) NPAs in the lung digestion and (D) hematocrit in WT mice with TRALI + 15-epi-LXA4 vs vehicle. *P < .05, **P < .01, and ***P < .001 vs vehicle groups. (E) Survival curves in mice with TRALI +15-epi-LXA4 vs vehicle. *P < .05 vs vehicle group. Data are mean ± SD of 10 animals per group.
Discussion
The main conclusions from this study are that (1) pulmonary platelet sequestration and activation, including intra-alveolar accumulation, are major features of ALI; (2) NPAs are dynamically formed in the lung microcirculation and sharply increase in the blood and alveolar compartments after injury; (3) ATL is required for aspirin-mediated protection in ALI, and ATL signals via Fpr2/3 on neutrophils and platelets to regulate the formation of NPA; and (4) in 2 experimental models of ALI, ATL treatment alone significantly reduces lung injury.
Platelets are capable of rapidly responding to inflammatory stimuli, releasing preformed mediators and even synthesizing immune mediators from mRNA transferred from megakaryocytes.28 Platelets and platelet microparticles29 can bind to leukocytes and the endothelium, influencing the function of these cells during inflammation. Indeed, using lung intravital imaging, we now show that the lung is a site of de novo NPA formation under basal conditions. The unique structural features of the lung microcirculation that influence neutrophil trafficking30 and the proposed role of the lung in thrombopoiesis31 may enhance these interactions. With inflammation, we found an early and rapid increase in dynamic NPA formation in the lung microcirculation, and many of these aggregates were ultimately detected in the alveolar spaces. Previous reports of NPA formation in inflammation have shown that neutrophils bound to the endothelium secondarily capture platelets,32,33 but our direct imaging of the lung microcirculation reveals that these aggregates are mobile and are eventually capable of migrating into the alveolar spaces. It has recently been described that the PF4-cre mouse is potentially leaky in nonmegakaryocyte lineages34 ; however, in the peripheral blood, we found that >99% of the PF4-cre–driven tdTomato+ events were platelet specific (CD41+).
There is a current lack of understanding of the regulation of LPA formation. Here, we propose that anti-inflammatory lipoxins and ATL specifically regulate this process. Lipoxins and ATL exert potent anti-inflammatory and pro-resolution bio-actions. In particular, they block chemotaxis and adherence to microvasculature, inhibit nuclear factor-κB activation in leukocytes, and block the release of proinflammatory cytokines such as interleukin-6, interleukin-8, and tumor necrosis factor-α.16,35-38 A potential limitation of our study is that microgram quantities of ATL were required to limit lung injury in our experiments, whereas others have reported that nanogram amounts of proresolving lipid mediators are sufficient.14 However, we treated with ATL at just 2 time points in our 48-hour LPS model, and more frequent dosing of ATL, which has a half-life of a few minutes, may have allowed for lower overall amounts. Also, we have not ruled out the contribution of other ASA-triggered lipid mediators, such as resolvins, but results from our pharmacologic experiments support a major role of ATL in mediating the protective effects of ASA.
Others have shown protective effects of lipoxins in acute inflammation. LXA4 and ATL were protective in an acid-induced ALI model through the modulation of neutrophil apoptosis.39 ATL was also protective in mice challenged with LPS through a heme-oxygenase1–dependent mechanism.40 Endogenous LXA4 modulates ischemia-reperfusion injury in the gut, and Fpr2/3−/− mice have more inflammation and injury compared with WT animals.41 In this same study, NPA formation was investigated using chimeric experiments to test whether platelet or neutrophil Fpr2/3 was more important in aggregate development, and neutrophil Fpr2/3 was determined to be critical.41 However, in our neutrophil-platelet aggregation assay, we found that neither neutrophil nor platelet Fpr2/3 is required for the formation of aggregates in response to LPS, but Fpr2/3 on both cells is required for lipoxin-mediated inhibition of NPA formation.
For the first time, we showed the presence of platelets in the alveolar spaces in ALI. Others have shown in experimental allergic inflammation that platelets migrate out of vessels and localize beneath airways42 or into the synovial spaces in rheumatoid arthritis.8 Platelets have also been observed by electron microscopy extravasating out of the lung vasculature in patients with ARDS.43 In our studies, many of the platelets were bound to neutrophils, and the dynamics of NPA migration into the alveolar spaces after LPS challenge seems to occur hours after the very early formation of NPAs in the lung vessels. More work is needed to determine the mechanisms of platelet transmigration in the lung, including the chemotactic stimuli for platelets, and whether transmigration is facilitated by tethering to neutrophils or occurs independently. Notably, our assays could have detected intact platelets or platelet microparticles in the alveolar spaces, and the specific role of platelet microparticles in lung inflammation should be addressed in future studies.
We previously reported that aspirin is protective in a 2-event model of TRALI by decreasing platelet sequestration and activation and neutrophil extracellular traps formation.2,21 Here, we found that ATL and ALX were required for the protective effects of aspirin in TRALI. Indeed, treatment of mice with ATL alone yielded significant protection from TRALI. These results have important clinical implications for patients with ARDS including transfused, critically ill patients. Platelet depletion is impractical, as is aspirin therapy in many critically ill patients, but treatment with ATL, or perhaps other anti-inflammatory and proresolving mediators,44 is an attractive approach. Further, the measurement of LPA in patients with ARDS and other critical illnesses could be a useful biomarker of inflammation and could be measured serially to assess therapeutic responses to treatment with pro-resolving lipid mediators.
In summary, we showed in 2 experimental models of ALI that aspirin-triggered lipoxin is a powerful inhibitor of neutrophil- and platelet-mediated lung inflammation and injury. Ironically, surface contact between neutrophil and platelets yields transcellular production of lipoxins and also leukotrienes,45 and our results indicate that signaling through ALX on neutrophils and platelets in turn regulates their tethering during inflammation. Aspirin is likely exerting its therapeutic benefit through a combination of inhibiting the production of proinflammatory eicosanoids and triggering the formation of proresolving lipid mediators.46 Even low-dose aspirin administered to healthy volunteers is capable of producing bioactive levels of ATL,47 and a clinical trial (ClinicalTrials.gov identifier #NCT01504867) is underway randomizing patients at risk for ARDS to aspirin or placebo. ATL and potentially other pro-resolving lipid mediators are attractive therapeutic options for patients with ARDS: a life-threatening syndrome that lacks any effective pharmacotherapies.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank K. Corbin and H. Pinkard at the University of California, San Francisco Biological Imaging Development Center for assistance with the intravital lung microscopy experiments and Rod Flower at the William Harvey Research Institute, St. Barts, and the London School of Medicine and Dentistry for providing the Fpr2/3−/− mice.
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute grants R01 HL107386 (M.R.L.) and P01 HL024136 (M.F.K).
Authorship
Contribution: G.O.-M. designed and performed the experiments, analyzed the results, and prepared the manuscript; B.M. performed experiments and edited the manuscript; A.B. assisted with the intravital lung imaging experiments and edited the manuscript; M.H. assisted with the intravital lung imaging experiments and edited the manuscript; M.F.K. assisted with the intravital lung imaging experiments and edited the manuscript; and M.R.L. designed and assisted with the experiments, analyzed the results, provided funding, and prepared and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark R. Looney, 513 Parnassus Ave, HSE 1355A, San Francisco, CA 94143-0130; e-mail: mark.looney@ucsf.edu.