Key Points
SOX11 mediates regulation of angiogenesis via the PDGFA signaling pathway in MCL.
SOX11-dependent increased angiogenesis contributes to a more aggressive MCL phenotype.
Abstract
SOX11 is overexpressed in several solid tumors and in the vast majority of aggressive mantle cell lymphomas (MCLs). We have recently proven that SOX11 silencing reduces tumor growth in a MCL xenograft model, consistent with the indolent clinical course of the human SOX11-negative mantle cell lymphoma (MCL). However, the direct oncogenic mechanisms and downstream effector pathways implicated in SOX11-driven transformation remain poorly understood. Here, we observed that SOX11-positive xenograft and human primary MCL tumors overexpressed angiogenic gene signatures and had a higher microvascular density compared with their SOX11-negative counterparts. Conditioned media of SOX11-positive MCL cell lines induced in vitro endothelial cell proliferation, migration, tube formation, and activation of downstream angiogenic pathways. We identified PDGFA as a SOX11 direct target gene upregulated in MCL cells whose inhibition impaired SOX11-enhanced in vitro angiogenic effects on endothelial cells. In addition, platelet-derived growth factor A (PDGFA) was overexpressed in SOX11-positive but not in SOX11-negative MCL. In vivo, imatinib impaired tumor angiogenesis and lymphoma growth in SOX11-positive MCL xenograft tumors. Overall, our results demonstrate a prominent role for SOX11 as a driver of proangiogenic signals in MCL, and highlight the SOX11-PDGFA axis as a potential therapeutic target for the treatment of this aggressive disease.
Introduction
Mantle cell lymphoma (MCL) is an aggressive lymphoid neoplasia derived from mature B cells genetically characterized by the presence of the t(11;14)(q13;q32) translocation causing cyclin D1 overexpression.1 Furthermore, other secondary genetic alterations also contribute to the development and aggressiveness of MCL.2 However, recent studies have identified a subset of MCL with indolent clinical behavior that tends to present with leukemic disease instead of extensive nodal infiltration and patients may not need chemotherapy for long periods.3-5 Recently, molecular studies have identified SOX11 (SRY [sex determining region-Y]-box11), as one of the best characterized discriminatory genes between these 2 clinical subtypes of MCL tumors.6
SOX11, together with SOX4 and SOX12, belongs to the subgroup C of the SOX gene family encoding for transcription factors which play a critical role in embryonic development and cell differentiation.7,8 SOX11 plays an important role in the regulation of neuronal cell survival and neurite growth, and is highly expressed in different central nervous system malignancies, solid tumors, aggressive MCL, and at lower levels in a subgroup of Burkitt and lymphoblastic lymphomas.9-14 However, the oncogenic mechanisms of SOX11 contributing to the development and progression of these tumors are largely unknown.
We have recently demonstrated the in vivo tumorigenic potential of SOX11 in a MCL xenograft model. SOX11 blocks the terminal B-cell differentiation through direct positive regulation of PAX515 but the specific mechanisms regulated by SOX11 promoting the oncogenic and rapid tumor growth of aggressive MCL still remain to be elucidated.
To further characterize the potential oncogenic mechanisms regulated by SOX11 in MCL, we have investigated the gene and protein expression profiling of SOX11-positive and -knockdown MCL xenograft tumors, cell lines, and primary SOX11-positive and SOX11-negative MCL. We have identified that SOX11 modulates angiogenesis in MCL, and this mechanism is mediated by the upregulation of several proangiogencic factors, principally platelet-derived growth factor A (PDGFA). The inhibition of the PDGFA pathway not only impairs angiogenic development both in vitro and in vivo but also MCL tumor growth in vivo, offering a promising novel therapeutic strategy for the treatment of aggressive MCL.
Methods
Cell lines and primary tumors
Three well-characterized SOX11-expressing MCL cell lines (Z138, GRANTA519, and JEKO1) were used for SOX11 silencing, xenograft experiments, western blot (WB), chromatin immunoprecipitation–quantitative polymerase chain reaction (ChIP-qPCR), in vitro experiments, and immunohistochemical studies. These 3 cell lines carry the t(11;14) and cyclin D1 overexpression.16 Human umbilical vein endothelial cells (HUVECs) were used for in vitro angiogenesis experimental studies.
Microarray gene expression profiling (GEP) data from 38 primary MCL tumors, 16 SOX11-expressing, and 22 SOX11-negative were used for gene set enrichment analyses (GSEAs) (GSE36000).17 In addition, 17 splenic MCL, 8 SOX11-expressing, and 9 SOX11-negative, were also investigated for the expression of PDGFA, CD31, CD34 by immunohistochemistry. Details on cell culture and human primary tumor information are provided in supplemental Methods (available on the Blood Web site).
Xenograft mouse model
With the use of a protocol approved by the animal testing ethical committee of the University of Barcelona, CB17-severe combined immunodeficient (CB17-SCID) mice (Charles River Laboratories) were subcutaneously inoculated into their lower dorsum with Z138, JEKO1, and GRANTA519 shControl, shSOX11.1, and shSOX11.3 cells as previously described,15 generating SOX11-positive and -knockdown xenograft tumors (shControl, shSOX11.1, and shSOX11.3, respectively).
When tumor volumes reached 75 to 100 mm3, mice were randomized to receive imatinib (LC Laboratories) dissolved in phosphate-buffered saline (PBS; Roche Applied Science) at 45 mg/kg or vehicle PBS twice daily by intraperitoneal (IP) injections for 2 weeks. Animals were euthanized according to institutional guidelines, and tumor xenografts were paraffin embedded on saline-coated slides in a fully automated immunostainer (Bond Max; Vision Biosystems) for immunohistochemical analysis and on Tissue-Tek OCT (Sakura) frozen in dry ice and stored at −80°C for RNA and protein extraction.
GEP and GSEA analyses
To identify oncogenic pathways related to SOX11 high expression in MCL, we performed GSEA18 on expression data sets derived from MCL primary tumors (GSE36000),17 in vitro SOX11 silencing experiments (GSE34763),15 and MCL xenografts (GEP derived from Z138 shControl, n = 4; shSOX11.1, n = 5; and shSOX11.3, n = 5). Experimental details on RNA extraction and GEP from xenograft tumors and GSEA are provided in supplemental Methods.
Angiogenesis proteome profiler antibody array
Total protein extracts from in vitro and xenograft SOX11-positive and SOX11-negative cells were used to study the human angiogenesis proteome profiler antibody array (R&D Systems) following the manufacturer’s protocol. Experimental details on protein extraction and proteome array are provided in supplemental Methods.
HUVECs in vitro angiogenesis studies
HUVECs were resuspended in RPMI + 10% fetal bovine serum (FBS) or SOX11-positive or SOX11-negative conditioned media (CM) and after the corresponding incubation time, their tube formation, proliferation, and migration were analyzed. Experimental details are provided in supplemental Methods.
HUVEC WB and PDGF pathway phosphospecific antibody array experiments
HUVECs were incubated in RPMI + 10% FBS or SOX11-positive or SOX11-negative CM for 3 hours, and WB experiments19 and PDGF pathway phosphospecific antibody array were performed. Experimental details are provided in supplemental Methods.
Custom human quantibody array
SOX11-positive and SOX11-negative CM were incubated in the protein arrays for the quantification of the secreted levels of 8 proangiogenic factors (ANG, ANGPT1, ANGPT2, FGF1, FGF2, PDGFA, PDGFB, and VEGF) according to the manufacturer’s protocol (RayBiotech). Experimental details are provided in supplemental Methods.
ChIP and ChIP-qPCR experiments
ChIP was carried out using the HighCell #ChIP kit (Diagenode). Briefly, Z138 and JEKO1 MCL cell lines were fixed and then sonicated with Biorupter sonicator (Diagenode). SOX11 antibody was used to immunoprecipitate protein-DNA complexes, and after immunoprecipitation, DNA was purified and quantified.
Primers for ChIP-qPCR were designed for SOX11 ChIP-enriched genomic DNA, and SOX11-ChIP DNA and 1:100 diluted input samples were analyzed in duplicate by qRT-PCR regions. Experimental details on ChIP, primers for ChIP-qPCR, and ChIP gene ontology (GO) categories analysis are provided in supplemental Methods.
Reporter plasmid constructs and luciferase assay
The SOX11-binding site to the regulatory region of PDGFA gene was amplified, cloned in front of a minimal promoter luciferase reporter vector pGL4.23[luc2/minP] (Promega), and used for transient cotransfection experiments and luciferase activity assays in HEK293 cells. Experimental details are provided in supplemental Methods.
In vitro PDGFA inhibition experiments
HUVECs were treated with 1µM imatinib or 10 µg/mL neutralizing antibody against human PDGFR-α (MAB322; R&D Systems) for 1 hour, and resuspended in RPMI + 10% FBS or SOX11-positive or SOX11-negative CM. HUVEC tube formation and migration were studied as previously described. Experimental details are provided in supplemental Methods.
Immunohistochemical staining and MVD quantification
Immunohistochemical staining studies were performed as previously described.14,20 CD31 or CD34-positive microvessel density (MVD) areas were calculated as the sum of areas of MVD (μm2) evaluated divided by the total area of the core analyzed (μm2) as previously described.21 Experimental details on immunohistochemical staining, antibodies used, and MVD quantification are provided in supplemental Methods.
Statistical analysis
Data are represented as mean ± standard deviation (SD) of 3 independent experiments. Statistical tests were performed using SPSS, v16.0 software (SPSS). Comparison between 2 groups of samples was evaluated by independent sample Student t test, and results were considered statistically significant when P < .05.
Results
SOX11 induces the expression of proangiogenic factors and promotes angiogenesis in MCL xenograft tumors
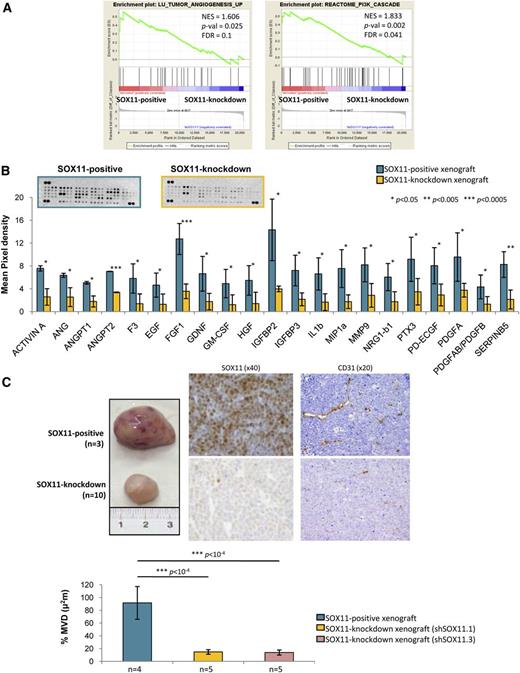
To investigate the mechanisms regulated by SOX11 promoting MCL tumor growth, we first compared the GEPs of SOX11-positive and SOX11-knockdown xenograft tumors.15 GSEA showed that SOX11-positive xenografts were significantly enriched in gene signatures related to tumor angiogenesis among other tumor microenvironment prosurvival signals, including phosphatidylinositol 3-kinase cascade (Figure 1A, Table 1). At the protein level, SOX11-positive xenografts had a significantly increased expression of 21 proangiogenic factors compared with SOX11-knockdown xenografts including ANG, MMP9, PDGFA, and PDGFAB/PDGFB (Figure 1B). Moreover, SOX11-positive xenografts displayed higher CD31-positive MVD areas than SOX11-knockdown ones (90% ± µm2 vs 15% ± µm2, respectively, P < 1 × 10−4) (Figure 1C). Together, these results suggest that SOX11 may facilitate tumor growth by upregulating different proangiogenic factors and enhancing MCL tumor angiogenesis in vivo.
SOX11 induces the expression of proangiogenic factors and promotes angiogenesis in xenograft MCL-derived tumors. (A) GSEA analysis on expression data set from SOX11-positive and SOX11-knockdown xenograft tumors showing enriched gene sets related to angiogenesis pathways. NES, P-val, and FDR are represented. Statistical significance is considered when FDR <0.2. (B) Top panel, Representative images showing human-specific angiogenesis antibody array membranes incubated with protein extracts from SOX11-positive (n = 2) and SOX11-knockdown (n = 2) xenograft tissue lysates. Bottom panel, Quantification of the mean pixel densities showing 21 angiogenic proteins significantly upregulated in SOX11-positive compared with SOX11-knockdown xenografts. (C) Top panel, Macroscopic appearance and consecutive histological sections from representative SOX11-positive and SOX11-knockdown xenografts (Z138 shControl, n = 3; shSOX11.1, n = 5; and shSOX11.3, n = 5) stained with a specific antibody anti-human SOX11 (×40) and a specific antibody anti-mouse CD31 (×20). Bottom panel, Density (% of CD31-positive microvessel areas) of CD31-positive MVD areas in SOX11-positive and SOX11-knockdown tumors delineated by the presence of CD31-positive staining. Bar plot represents the mean percentage ± SD. P-val are shown. The significance of difference was determined by the independent samples Student t test. FDR, false discovery rate; NES, normalized enrichment score; P-val, P value.
SOX11 induces the expression of proangiogenic factors and promotes angiogenesis in xenograft MCL-derived tumors. (A) GSEA analysis on expression data set from SOX11-positive and SOX11-knockdown xenograft tumors showing enriched gene sets related to angiogenesis pathways. NES, P-val, and FDR are represented. Statistical significance is considered when FDR <0.2. (B) Top panel, Representative images showing human-specific angiogenesis antibody array membranes incubated with protein extracts from SOX11-positive (n = 2) and SOX11-knockdown (n = 2) xenograft tissue lysates. Bottom panel, Quantification of the mean pixel densities showing 21 angiogenic proteins significantly upregulated in SOX11-positive compared with SOX11-knockdown xenografts. (C) Top panel, Macroscopic appearance and consecutive histological sections from representative SOX11-positive and SOX11-knockdown xenografts (Z138 shControl, n = 3; shSOX11.1, n = 5; and shSOX11.3, n = 5) stained with a specific antibody anti-human SOX11 (×40) and a specific antibody anti-mouse CD31 (×20). Bottom panel, Density (% of CD31-positive microvessel areas) of CD31-positive MVD areas in SOX11-positive and SOX11-knockdown tumors delineated by the presence of CD31-positive staining. Bar plot represents the mean percentage ± SD. P-val are shown. The significance of difference was determined by the independent samples Student t test. FDR, false discovery rate; NES, normalized enrichment score; P-val, P value.
Conditioned media from SOX11-positive cell lines promote in vitro angiogenesis
We next analyzed the effects of the CM derived from SOX11-positive and -silenced MCL cell lines on several angiogenic responses of endothelial cells in vitro. We first performed tube formation assays using cultured HUVECs on Matrigel matrix.22 As shown in Figure 2A, SOX11-positive CM significantly promoted a prominent increase in tube formation compared with CM from SOX11-negative cells and RPMI + 10% FBS. Conversely, SOX11-negative CM did not have an effect on tube formation compared with RPMI + 10% FBS. Concordantly, HUVECs displayed significantly increased proliferation (Figure 2B) and migration (Figure 2C) toward SOX11-positive CM of the 3 cell lines compared with SOX11-negative CM. We next confirmed that SOX11-positive CM media induced phosphorylation of Akt, FAK, and Erk1/2 compared with SOX11-negative CM or RPMI + 10% FBS whereas basal levels of these proteins did not change (Figure 2D). These results suggest that SOX11-positive CM may contain soluble factors that promote endothelial tube formation, proliferation, migration, and activation of signaling pathways required for angiogenesis.
SOX11 promotes endothelial tube formation, cell proliferation and migration, and activation of angiogenic signaling cascades in vitro. (A) Top panel, Representative images showing the capillary tube formation by HUVECs growing on Matrigel matrix for 6 hours in the presence of control RPMI + 10% FBS, SOX11-positive or SOX11-negative CM on Matrigel matrix. Bottom panel, Quantification of the number of branching points representing tube formation by HUVECs cultured for 6 hours in RPMI + 10% FBS or SOX11-positive and SOX11-negative CM from Z138, JEKO1, and GRANTA519 MCL cell lines. SOX11-positive CM significantly promoted a prominent increase in tube formation compared with CM from SOX11-negative cells (126 vs 89 branching points, P = .0002, 121 vs 100 branching points, P = .009, and 102 vs 70 branching points, P < 1 × 10−4 in Z138, JEKO1, and GRANTA519) and RPMI + 10% FBS (126 vs 76 branching points, P < 1 × 10−4, 121 vs 76 branching points, P < 1 × 10−4, and 102 vs 76 branching points, P < 1 × 10−4 in Z138, JEKO1, and GRANTA519). Conversely, SOX11-negative CM did not have an effect on tube formation compared with RPMI + 10% FBS (89 vs 76 branching points, P = .2, 70 vs 76 branching points, P = .3 in Z138 and GRANTA519). (B) Kinetics of HUVEC growth in SOX11-positive CM (blue) or SOX11-negative CM (yellow) derived from SOX11-positive and SOX11-silenced Z138 (solid line) and JEKO1 (dashed line) MCL cell lines. Graph showing the optical density (620 nm) after staining the HUVECs with 0.2% crystal violet and measured at 620-nm wavelengths at the indicated time points (1, 3, and 5 days). (C) Graph displaying the percentage of migratory HUVEC cells toward SOX11-positive and SOX11-negative CM from Z138, JEKO1, and GRANTA519 MCL cell lines, relative to the percentage of migratory HUVEC cells toward RPMI + 10% FBS. HUVECs displayed significantly increased migration toward SOX11-positive CM cell lines (±90% migration) compared with SOX11-negative CM (±30%-70% migration, in Z138 and GRANTA519 [P < 1 × 10−4] and JEKO1 [P = .009]). (D) Left panel, WB experiments showing expression levels of basal and phosphorylated forms of FAK, Akt, and Erk1/2 proteins in HUVECs cultured in RPMI + 10% FBS or SOX11-positive and SOX11-negative CM from Z138 and GRANTA519 MCL cell lines for 3 hours. GAPDH was used as a loading control. Right panel, Fold change differences of phosphorylated forms of FAK, Akt, and Erk1/2 proteins in HUVECs cultured in RPMI + 10% FBS or SOX11-positive and SOX11-negative CM from Z138 and GRANTA519 MCL cell lines for 3 hours, correlated by quantification of GAPDH expression levels. Bar plot represents the mean percentage ± SD, n = 3. P-val is shown. The significance of difference was determined by independent samples Student t test. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
SOX11 promotes endothelial tube formation, cell proliferation and migration, and activation of angiogenic signaling cascades in vitro. (A) Top panel, Representative images showing the capillary tube formation by HUVECs growing on Matrigel matrix for 6 hours in the presence of control RPMI + 10% FBS, SOX11-positive or SOX11-negative CM on Matrigel matrix. Bottom panel, Quantification of the number of branching points representing tube formation by HUVECs cultured for 6 hours in RPMI + 10% FBS or SOX11-positive and SOX11-negative CM from Z138, JEKO1, and GRANTA519 MCL cell lines. SOX11-positive CM significantly promoted a prominent increase in tube formation compared with CM from SOX11-negative cells (126 vs 89 branching points, P = .0002, 121 vs 100 branching points, P = .009, and 102 vs 70 branching points, P < 1 × 10−4 in Z138, JEKO1, and GRANTA519) and RPMI + 10% FBS (126 vs 76 branching points, P < 1 × 10−4, 121 vs 76 branching points, P < 1 × 10−4, and 102 vs 76 branching points, P < 1 × 10−4 in Z138, JEKO1, and GRANTA519). Conversely, SOX11-negative CM did not have an effect on tube formation compared with RPMI + 10% FBS (89 vs 76 branching points, P = .2, 70 vs 76 branching points, P = .3 in Z138 and GRANTA519). (B) Kinetics of HUVEC growth in SOX11-positive CM (blue) or SOX11-negative CM (yellow) derived from SOX11-positive and SOX11-silenced Z138 (solid line) and JEKO1 (dashed line) MCL cell lines. Graph showing the optical density (620 nm) after staining the HUVECs with 0.2% crystal violet and measured at 620-nm wavelengths at the indicated time points (1, 3, and 5 days). (C) Graph displaying the percentage of migratory HUVEC cells toward SOX11-positive and SOX11-negative CM from Z138, JEKO1, and GRANTA519 MCL cell lines, relative to the percentage of migratory HUVEC cells toward RPMI + 10% FBS. HUVECs displayed significantly increased migration toward SOX11-positive CM cell lines (±90% migration) compared with SOX11-negative CM (±30%-70% migration, in Z138 and GRANTA519 [P < 1 × 10−4] and JEKO1 [P = .009]). (D) Left panel, WB experiments showing expression levels of basal and phosphorylated forms of FAK, Akt, and Erk1/2 proteins in HUVECs cultured in RPMI + 10% FBS or SOX11-positive and SOX11-negative CM from Z138 and GRANTA519 MCL cell lines for 3 hours. GAPDH was used as a loading control. Right panel, Fold change differences of phosphorylated forms of FAK, Akt, and Erk1/2 proteins in HUVECs cultured in RPMI + 10% FBS or SOX11-positive and SOX11-negative CM from Z138 and GRANTA519 MCL cell lines for 3 hours, correlated by quantification of GAPDH expression levels. Bar plot represents the mean percentage ± SD, n = 3. P-val is shown. The significance of difference was determined by independent samples Student t test. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
SOX11 drives transcriptional activation of PDGFA
To identify the angiogenic factors expressed and secreted by SOX11-positive cells, we first performed a GSEA of the differential gene expression data sets of our MCL cell lines15 and observed that, similarly to the xenograft tumors, SOX11-positive cell lines were also enriched in gene signatures related to tumor angiogenesis (Figure 3A, Table 1). We next investigated the expression levels of several angiogenic factors in the protein extracts of SOX11-positive and SOX11-silenced cells. Although the number of proangiogenic proteins differentially expressed between SOX11-positive and SOX11-silenced cell lines was much lower than in the tumor xenograft lysates, SOX11-positive cells still expressed significantly higher levels of the proangiogenic factors Activin A, ANGPT2, PDGFA, and VEGF compared with SOX11-silenced ones (Figure 3B).
SOX11 binds to regulatory regions of PDGFA and positively regulates its transcription. (A) GSEA analysis on expression data set from SOX11-positive and SOX11-silenced MCL cell lines showing enriched gene sets related to angiogenesis pathways. NES, P-val, and FDR are represented. Statistical significance is considered when FDR < 0.2. (B) Top panel, Image showing human-specific angiogenesis antibody array membranes incubated with protein lysates from Z138 SOX11-positive (n = 2) and SOX11-silenced (n = 2) MCL cell lines. Bottom panel, Quantification of mean pixel density of the proangiogenic factors Activin A, ANGPT2, PDGFA, and VEGF comparing SOX11-positive and SOX11-silenced MCL cell lines. (C) Graph displaying the relative levels of PDGFA secreted by SOX11-positive and SOX11-silenced CM derived from Z138 and GRANTA519 MCL cell lines. Concentration was analyzed by a quantibody human angiogenesis array. Results are shown as relative percentage of PDGFA secretion compared with SOX11-positive CM. (D) Graph displaying the GO term results obtained from DAVID functional annotation tool of the high confidence SOX11-bound genes identified by ChIP-chip experiments (GSE3502).15 The 6 most significant biological process GO terms and their gene count, enrichment score, and P-val are shown. Underlined is the blood vessel development GO biological term. (E) ChIP-qPCR analysis of specific binding of SOX11 to the regulatory regions of PDGFA in JEKO1 and Z138 MCL cell lines. Binding to PAX5 was used as a positive internal control.15 Relative DNA enrichment was measured by qPCR using specific primers for the respective regulatory regions (see “Methods”), and is displayed as fold enrichment relative to their respective input chromatin. Purple bars represent ChIP-qPCR enrichment of the SOX11 pull-down (SOX11-ChIPed DNA) while gray bars represent the negative control (IgG-ChIPed DNA). (F) Luciferase assays in transient cotransfections of PAX5-GL4.23 Luc and PDGFA-GL4.23 Luc with SOX11 full-length (pcDNA3-SOX11) and the truncated SOX11 proteins (pcDNA3-SOX11ΔHMG) expression vectors in HEK293 cells. Results are shown as the percentage of fold induction relative to luciferase activity in cotransfection with the empty vector (pcDNA3-Ǿ). Bar plot represents the mean percentage ± SD of 3 independent experiments. P-val are shown. The significance of difference was determined by independent samples Student t test.
SOX11 binds to regulatory regions of PDGFA and positively regulates its transcription. (A) GSEA analysis on expression data set from SOX11-positive and SOX11-silenced MCL cell lines showing enriched gene sets related to angiogenesis pathways. NES, P-val, and FDR are represented. Statistical significance is considered when FDR < 0.2. (B) Top panel, Image showing human-specific angiogenesis antibody array membranes incubated with protein lysates from Z138 SOX11-positive (n = 2) and SOX11-silenced (n = 2) MCL cell lines. Bottom panel, Quantification of mean pixel density of the proangiogenic factors Activin A, ANGPT2, PDGFA, and VEGF comparing SOX11-positive and SOX11-silenced MCL cell lines. (C) Graph displaying the relative levels of PDGFA secreted by SOX11-positive and SOX11-silenced CM derived from Z138 and GRANTA519 MCL cell lines. Concentration was analyzed by a quantibody human angiogenesis array. Results are shown as relative percentage of PDGFA secretion compared with SOX11-positive CM. (D) Graph displaying the GO term results obtained from DAVID functional annotation tool of the high confidence SOX11-bound genes identified by ChIP-chip experiments (GSE3502).15 The 6 most significant biological process GO terms and their gene count, enrichment score, and P-val are shown. Underlined is the blood vessel development GO biological term. (E) ChIP-qPCR analysis of specific binding of SOX11 to the regulatory regions of PDGFA in JEKO1 and Z138 MCL cell lines. Binding to PAX5 was used as a positive internal control.15 Relative DNA enrichment was measured by qPCR using specific primers for the respective regulatory regions (see “Methods”), and is displayed as fold enrichment relative to their respective input chromatin. Purple bars represent ChIP-qPCR enrichment of the SOX11 pull-down (SOX11-ChIPed DNA) while gray bars represent the negative control (IgG-ChIPed DNA). (F) Luciferase assays in transient cotransfections of PAX5-GL4.23 Luc and PDGFA-GL4.23 Luc with SOX11 full-length (pcDNA3-SOX11) and the truncated SOX11 proteins (pcDNA3-SOX11ΔHMG) expression vectors in HEK293 cells. Results are shown as the percentage of fold induction relative to luciferase activity in cotransfection with the empty vector (pcDNA3-Ǿ). Bar plot represents the mean percentage ± SD of 3 independent experiments. P-val are shown. The significance of difference was determined by independent samples Student t test.
To determine whether MCL cells secreted these factors in vitro, we analyzed SOX11-CM using a quantibody angiogenesis array and observed that SOX11-positive CM had significantly increased levels of PDGFA secretion compared with SOX11-negative CM (Figure 3C). No other proangiogenic factors analyzed (PDGFB, ANG, ANGPT1, ANGPT2, VEGF, FGF1, or FGF2) were detected in any MCL CM.
We performed a GO term analysis of our previous genome-wide promoter ChIP-chip study,15 which showed that blood vessel development was one of the most significant biological processes overrepresented among the SOX11-bound genes (Figure 3D). However, among the proangiogenic factors overexpressed in the SOX11-positive cell lines (Figure 3B), PDGFA was the only one identified as a SOX11-bound gene by ChIP experiments, with no significant binding to Activin A, ANGPT2, or VEGF regulatory regions. Following on these results, we validated the specific binding of SOX11 to the regulatory region of PDGFA by ChIP-qPCR experiments. We observed a ±twofold and ±fourfold enrichment for PDGFA in Z138 and JEKO1, respectively, demonstrating that SOX11 directly binds to regulatory regions of PDGFA (Figure 3E).
Luciferase reporter assays with a PDGFA regulatory construct encompassing the SOX11 binding site showed a significant threefold induction (P < 1 ×10−4) in luciferase activity upon coexpression of SOX11 but not with an inactive truncated SOX11 protein lacking the High Mobility Group (HMG) domain (ΔHMGSOX11). Similar results were obtained with a PAX5-enhancer-luciferase reporter vector,15 used as a positive control (Figure 3F).
Together, these findings verify the positive transcriptional effect of SOX11 on the PDGFA regulatory region and suggest that PDGFA may be the major mediator of angiogenic in SOX11-positive MCLs.
Inhibition of the PDGFA signaling pathway on endothelial cells impairs SOX11-enforced angiogenesis in MCL
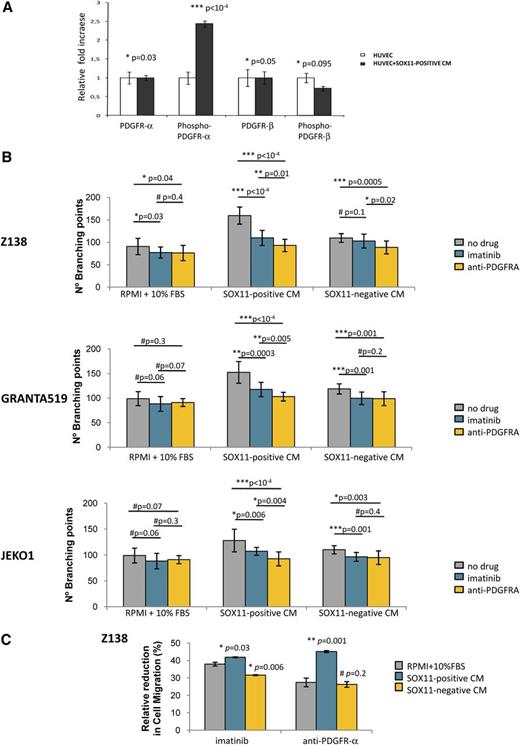
To validate the involvement of PDGFA in promoting angiogenesis in MCL, we analyzed the phosphorylation level of endogenous PDGFR-α in HUVECs upon incubation with Z138 SOX11-positive CM. We observed a significant 2.4-fold increase (P < 1 × 10−4) in PDGFR-α but not in PDGFR-β phosphorylation when HUVECs were cultured in Z138 SOX11-positive CM compared with RPMI + 10% FBS (Figure 4A), demonstrating the paracrine activation of the PDGFA/PDGFR-α angiogenic pathway on endothelial cells in MCL. Furthermore, we treated HUVECs with imatinib, a tyrosine kinase inhibitor of PDGFR, or a neutralizing antibody against human PDGFR-α, and then performed tube formation and migration assays in the presence of RPMI + 10% FBS or SOX11-positive CM. The tube formation induced by SOX11-positive CM of Z138, GRANTA519, and JEKO1 was significantly decreased when HUVECs were pretreated with imatinib or the neutralizing PDGFR-α antibody (Figure 4B). Both pretreatments also caused a significant reduction on HUVEC migration toward SOX11-positive CM when compared with no drug treatment (Figure 4C).
PDGFA inhibition on endothelial cells impairs SOX11-enforced angiogenesis in MCL. (A) Graph showing the relative fold change expression levels of the basal and phosphorylated forms of PDGFRα and PDGFRβ by HUVEC cells upon 3-hour incubation with RPMI + 10% FBS (HUVEC) or Z138 SOX11-positive CM (HUVEC+SOX11-positive CM). Results are shown as fold increase relative to the corresponding expression levels by the HUVECs incubated with RPMI + 10% FBS. (B) Graphs showing number of branching points representing tube formation by HUVECs. HUVECs were pretreated with control PBS (gray), imatinib (blue), or a neutralizing antibody anti-PDGFRα (yellow) for 1 hour, and then incubated for 6 hours with RPMI + 10% FBS, SOX11-positive or SOX11-negative CM from Z138, GRANTA519, and JEKO1 MCL cell lines. (C) Graph displaying the relative perctage reduction in migration of HUVECs. HUVECs were pretreated with control PBS, imatinib, or a neutralizing antibody anti-PDGFRα for 1 hour, and then allowed to migrate toward RPMI + 10% FBS (gray), SOX11-positive CM (blue) or SOX11-negative CM (yellow) from Z138 MCL cell line. Upon overnight incubation, migratory cells were quantified and represented as the percentage of migration HUVECs compared with no drug (control PBS) treatment. Bar plot represents the mean percentage ± SD of 3 independent experiments. P-val are shown. The significance of difference was determined by independent samples Student t test.
PDGFA inhibition on endothelial cells impairs SOX11-enforced angiogenesis in MCL. (A) Graph showing the relative fold change expression levels of the basal and phosphorylated forms of PDGFRα and PDGFRβ by HUVEC cells upon 3-hour incubation with RPMI + 10% FBS (HUVEC) or Z138 SOX11-positive CM (HUVEC+SOX11-positive CM). Results are shown as fold increase relative to the corresponding expression levels by the HUVECs incubated with RPMI + 10% FBS. (B) Graphs showing number of branching points representing tube formation by HUVECs. HUVECs were pretreated with control PBS (gray), imatinib (blue), or a neutralizing antibody anti-PDGFRα (yellow) for 1 hour, and then incubated for 6 hours with RPMI + 10% FBS, SOX11-positive or SOX11-negative CM from Z138, GRANTA519, and JEKO1 MCL cell lines. (C) Graph displaying the relative perctage reduction in migration of HUVECs. HUVECs were pretreated with control PBS, imatinib, or a neutralizing antibody anti-PDGFRα for 1 hour, and then allowed to migrate toward RPMI + 10% FBS (gray), SOX11-positive CM (blue) or SOX11-negative CM (yellow) from Z138 MCL cell line. Upon overnight incubation, migratory cells were quantified and represented as the percentage of migration HUVECs compared with no drug (control PBS) treatment. Bar plot represents the mean percentage ± SD of 3 independent experiments. P-val are shown. The significance of difference was determined by independent samples Student t test.
To determine whether PDGFR-α was expressed in MCL cells lines and primary tumors, we analyzed its messenger RNA (mRNA) levels in the GEP microarray data of our primary MCL tumors and MCL cell lines using as positive and negative references the previously published data of peripheral T-cell lymphoma (PTCL), a tumor known to express high levels of PDGFR-α, and the GEP of normal mature T cells, a subset of cells known to be negative for this receptor.23 We confirmed the high levels of PDGFR-α in the PTCL whereas all our primary MCL and MCL cell lines had undetectable levels similarly to normal T lymphocytes (supplemental Figure 1). These findings indicate the lack of expression of PDGFR-α in MCL cells and support the idea that the secreted PDGFA must have a paracrine effect on the endothelial cells rather than an autocrine action on the MCL cells. Altogether, these results demonstrate that the enhanced angiogenic activity of the SOX11-positive CM is mediated by PDGFA expression, and its inhibition impairs SOX11-promoted angiogenic effects on endothelial cells.
Human SOX11-positive primary MCL overexpress PDGFA and have increased angiogenesis
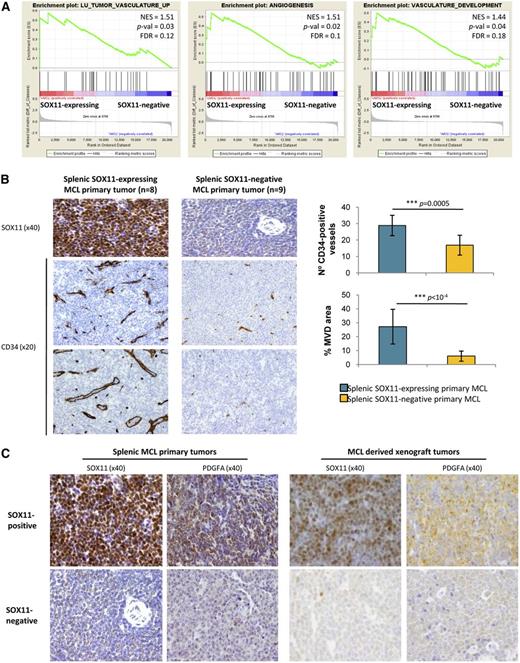
To determine whether SOX11 could be regulating angiogenesis in vivo, we analyzed gene expression signatures of primary human MCL samples. GSEA revealed that SOX11-expressing human primary tumors were also significantly enriched in signatures related to angiogenesis such as tumor vasculature, angiogenesis, vasculature development, regulation of angiogenesis, and tumor endothelial markers (Figure 5A, Table 1). We then analyzed the MVD in an independent series of human MCL primary tumors (Table 2) by staining splenic tissue sections of 8 SOX11-expressing and 9 SOX11-negative tumors for CD31 and CD34. Both antibodies provided similar results with SOX11-positive tumors showing a significantly higher number of vessels than SOX11-negative MCL (±30 microvessels per µm2 vs ±15 per µm2, respectively, P = .0005) and larger vascular areas (30% ± µm2 vs 5% ± µm2, P < 1 × 10−4) (Figure 5B). These results confirm that SOX11 may also promote angiogenesis in primary human MCL.
SOX11-positive MCL tumors have increased tumor angiogenesis network and PDGFA overexpression. (A) GSEA analysis on expression data sets from leukemic MCL primary tumors (16 SOX11-expressing and 22 SOX11-negative (GSE36000)17 showing enriched gene sets related to angiogenesis pathways. NES, P-val, and FDR are shown. Statistical significance is considered when FDR < 0.2. (B) Left panel, Histological sections from representative SOX11-expresing (n = 8) and SOX11-negative (n = 9) splenic MCL primary tumors stained with specific antibodies against human SOX11 (×40) and CD34 (×20). Right panel, Graph showing quantification of number of CD34-positive vessels and percentage of CD34-positive MVD areas of the splenic MCL primary tumors. Bar plot represents the mean percentage ± SD. P-val are shown. The significance of difference was determined by independent samples t test. (C) Representative histological sections from SOX11-positive and SOX11-negative tumors from splenic MCL primary samples (n = 8 and n = 9, respectively) and MCL xenograft tumors (n = 3 and n = 10, respectively) stained with a specific antibody anti-human SOX11 (×40) and PDGFA (×40).
SOX11-positive MCL tumors have increased tumor angiogenesis network and PDGFA overexpression. (A) GSEA analysis on expression data sets from leukemic MCL primary tumors (16 SOX11-expressing and 22 SOX11-negative (GSE36000)17 showing enriched gene sets related to angiogenesis pathways. NES, P-val, and FDR are shown. Statistical significance is considered when FDR < 0.2. (B) Left panel, Histological sections from representative SOX11-expresing (n = 8) and SOX11-negative (n = 9) splenic MCL primary tumors stained with specific antibodies against human SOX11 (×40) and CD34 (×20). Right panel, Graph showing quantification of number of CD34-positive vessels and percentage of CD34-positive MVD areas of the splenic MCL primary tumors. Bar plot represents the mean percentage ± SD. P-val are shown. The significance of difference was determined by independent samples t test. (C) Representative histological sections from SOX11-positive and SOX11-negative tumors from splenic MCL primary samples (n = 8 and n = 9, respectively) and MCL xenograft tumors (n = 3 and n = 10, respectively) stained with a specific antibody anti-human SOX11 (×40) and PDGFA (×40).
We next investigated whether PDGFA mRNA was also overexpressed in human primary SOX11-positive MCL using the data of our microarray GEP of 38 primary MCL tumors, 16 SOX11-expressing, and 22 SOX11-negative and found a significant 1.6-fold increased level in SOX11-positive tumors (P = .0303) (supplemental Figure 2). Concordantly, high PDGFA expression was immunohistochemically demonstrated on the tumor cells of 8 SOX11-expressing MCL whereas it was not detected in the tumor cells of 9 SOX11-negative tumors. These results were also confirmed in the SOX11-positive and SOX11-negative xenograft tumors (Figure 5C). Therefore, these findings indicate that SOX11 upregulates PDGFA expression in MCL both in vitro and in vivo, mediating the SOX11-enforced angiogenesis that may contribute to the aggressiveness of these tumors.
Imatinib reduces tumor growth and angiogenesis of SOX11-positive MCL xenograft tumors
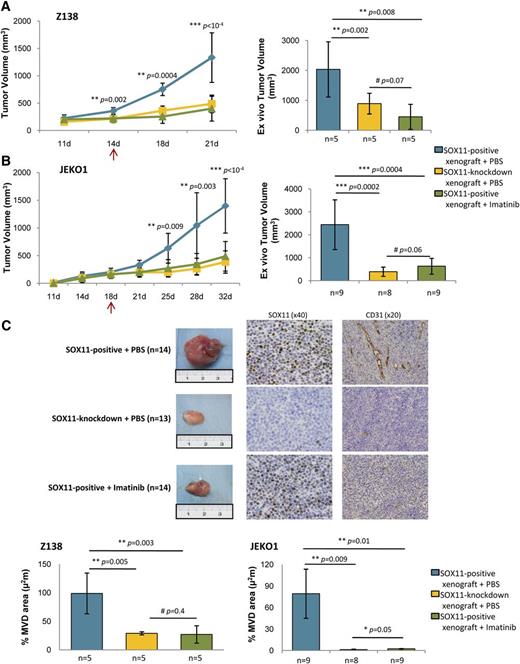
To demonstrate whether the in vitro inhibitory effect of imatinib on the SOX11-induced angiogenesis may also occur in vivo and, consequently, have an effect on tumor growth, we treated SOX11-positive and SOX11-negative MCL xenografts with imatinib or vehicle PBS and followed tumor growth up. In these experiments, imatinib treatment significantly suppressed tumor growth of the SOX11-positive xenografts compared with vehicle-treated controls (Figure 6A-B) bringing down the growth of SOX11-positive MCL xenograft to that of SOX11-knockdown tumors (Figure 6B).
Imatinib reduces MCL tumor growth and angiogenesis of SOX11-positive MCL xenograft tumors. (A-B) Left panel, Tumor growth represented by tumor volumes (mm3) at the indicated days PI of Z138 and JEKO1 SOX11-positive xenografts treated with IP injections of imatinib (n = 5, n = 9) (green) or vehicle PBS (n = 5, n = 9) (blue) and SOX11-knockdown xenografts treated with IP of vehicle PBS (n = 5, n = 8) (yellow). Right panel, Graph displaying tumor volumes (mm3) at the time of tissue harvest, comparing imatinib (green) vs vehicle PBS (blue) treated SOX11-positive xenografts and (yellow) vehicle PBS-treated SOX11-knockdown xenografts. Compared with vehicle PBS, imatinib treatment significantly suppressed tumor growth of the SOX11-positive xenografts observed during follow-up (400 mm3 vs 1330 mm3, P < 1 × 10−4, and 490 mm3 vs 1400 mm3, P < 1 × 10−4 in Z138 and JEKO1, respectively) and ex vivo measurements (450 mm3 vs 2000 mm3, P = .008, and 630 mm3 vs 2400 mm3, P = .0004 in Z138 and JEKO1, respectively). (C) Top panel, Macroscopic appearance and consecutive histological sections from representative SOX11-positive (n = 14) and SOX11-knockdown xenograft tumors (n = 13) upon imatinib or vehicle PBS treatment. Immunohistochemical stainings were performed with a specific antibody anti-human SOX11 (×40) and an anti-mouse CD31 (×20). Bottom panel, Graph displaying the percentage of density of CD31-positive MVD areas in Z138 and JEKO1 xenograft tumors in (green) imatinib or (blue) vehicle PBS-treated SOX11-positive xenografts and (yellow) vehicle PBS-treated SOX11-knockdown xenografts, delineated by the presence of CD31-positive staining. Bar plot represents the mean percentage ± SD. P-val are shown. The significance of difference was determined by independent sample Student t test. PI, postinoculation.
Imatinib reduces MCL tumor growth and angiogenesis of SOX11-positive MCL xenograft tumors. (A-B) Left panel, Tumor growth represented by tumor volumes (mm3) at the indicated days PI of Z138 and JEKO1 SOX11-positive xenografts treated with IP injections of imatinib (n = 5, n = 9) (green) or vehicle PBS (n = 5, n = 9) (blue) and SOX11-knockdown xenografts treated with IP of vehicle PBS (n = 5, n = 8) (yellow). Right panel, Graph displaying tumor volumes (mm3) at the time of tissue harvest, comparing imatinib (green) vs vehicle PBS (blue) treated SOX11-positive xenografts and (yellow) vehicle PBS-treated SOX11-knockdown xenografts. Compared with vehicle PBS, imatinib treatment significantly suppressed tumor growth of the SOX11-positive xenografts observed during follow-up (400 mm3 vs 1330 mm3, P < 1 × 10−4, and 490 mm3 vs 1400 mm3, P < 1 × 10−4 in Z138 and JEKO1, respectively) and ex vivo measurements (450 mm3 vs 2000 mm3, P = .008, and 630 mm3 vs 2400 mm3, P = .0004 in Z138 and JEKO1, respectively). (C) Top panel, Macroscopic appearance and consecutive histological sections from representative SOX11-positive (n = 14) and SOX11-knockdown xenograft tumors (n = 13) upon imatinib or vehicle PBS treatment. Immunohistochemical stainings were performed with a specific antibody anti-human SOX11 (×40) and an anti-mouse CD31 (×20). Bottom panel, Graph displaying the percentage of density of CD31-positive MVD areas in Z138 and JEKO1 xenograft tumors in (green) imatinib or (blue) vehicle PBS-treated SOX11-positive xenografts and (yellow) vehicle PBS-treated SOX11-knockdown xenografts, delineated by the presence of CD31-positive staining. Bar plot represents the mean percentage ± SD. P-val are shown. The significance of difference was determined by independent sample Student t test. PI, postinoculation.
To determine whether tumor growth reduction upon imatinib treatment was associated with impaired angiogenesis, tumor microvasculature was visualized by histologically staining imatinib- or PBS-treated xenografts with CD31. Compared with PBS treatment, administration of imatinib was strongly associated with a loss of microvasculature in the SOX11-positive xenografts, equaling the number of CD31-positive vessels and MVD areas to those observed in the SOX11-knockdown tumors (Figure 6C), suggesting that the abolishment in MVD was responsible for the tumor reduction observed in the imatinib-treated xenografts. Altogether, these results indicate that PDGFA inhibition could be effective to improve disease outcome of SOX11-positive MCL.
Discussion
Our study shows that increased tumor angiogenesis is a prominent feature of SOX11-positive MCL required for effective tumor growth, and provides evidences to support the inhibition of this mechanism as a new strategy for the management of aggressive MCL patients.
Angiogenesis deregulation is one of the hallmarks of cancer widely investigated in solid malignancies where it has been associated with neoplastic growth and progression.24-26 Recent studies have also revealed the pathogenic relevance and clinical impact of the “angiogenic switch” in lymphoid neoplasms but the mechanisms regulating this phenomenon are not well known.26,27 In this study, we have shown that the more aggressive SOX11-positive MCL xenografts were enriched in angiogenic gene signatures and displayed much larger microvascular density areas than their SOX11-negative counterparts, concurring with other observations that an increased MVD correlates with disease progression in lymphoid neoplasms.28,29 We therefore postulated that SOX11-positive MCL cells could promote a pronounced vasculature development and obtain an increased and deregulated blood supply that may contribute to their aggressive progression.
Interestingly, SOX11-positive CM promoted vascular tube formation, endothelial cell proliferation, and migration, and activation of relative angiogenic downstream pathways in HUVECs, indicating that SOX11-positive MCL cells secrete soluble factors that enhance signaling pathways related to cell migration and survival which are known to be activated by PDGFs in other cell types.30 Concordant with our results, a recent study in zebrafish has reported the involvement of SOX11b, the human ortholog of SOX11, in promoting sprouting angiogenesis of caudal vein plexus.31
The gene transcriptional profile of SOX11-positive MCL cell lines was enriched in signatures related to angiogenesis and expressed higher levels of several proangiogenic factors than SOX11-negative ones. On the other hand, the number of proangiogenic factors upregulated in SOX11-positive tumor xenografts was much larger than in the respective cell lines, highlighting the cross-talk between tumor and accessory cells of the microenvironment in the regulation of angiogenesis. Among the proangiogenic factors overexpressed by SOX11-positive MCL cell lines in vitro, we could only detect secreted PDGFA at high levels in their corresponding CM, and PDGFA was the only gene directly bound by SOX11 in our ChIP-chip study, strongly supporting the idea that this gene could be one of the main SOX11-dependent factors regulating angiogenesis in MCL.
PDGFA is a member of the PDGF family of proangiogenic factors which may participate in the development of a vascular tumor microenvironment through a direct effect on the endothelium but also indirectly by recruiting mesenchymal stromal cells that release additional angiogenic elements.32-35 The PDGF family consists of 5 isoforms that exert their cellular effects by binding to PDGFR-α or PDGFR-β with different affinities. PDGFA only binds to PDGFR-α, activating their downstream pathways involved in several oncogenic mechanisms including angiogenesis.36,37 Several studies have demonstrated that PDGFR-α is expressed by HUVECs and solid tumor endothelial cells,38,39 and here we demonstrated an increased phosphorylation of PDGFR-α but not PDGFR-β in HUVECs upon SOX11-positive CM incubation. Furthermore, we have demonstrated the activation of AKT, ERK, and FAK upon SOX11-positive CM incubation. The activation of these signaling pathways mediates proliferation, survival, and migration of endothelial responses required by the complex and multistep process of angiogenesis.
Pretreatment of HUVECs with imatinib or a specific neutralizing antibody against PDGFR-α inhibited their tube formation and migration promoted by the SOX11-positive CM. Although imatinib does not specifically target PDGFR kinases,40-42 the lack of expression of PDGFR-α and the 2 other target kinases c-KIT and BCR-ABL in MCL cell lines and primary tumors indicates that its inhibitory effects on HUVEC angiogenic capacities are due to a blockade of the PDGFA/PDGFR-α angiogenic pathway cascade. The similar inhibitory effects obtained using the specific neutralizing antibody against PDGFR-α corroborate the blockade of the PDGFA/PDGFR-α axis by imatinib impairing HUVEC angiogenic abilities and support the idea that PDGFA promotes angiogenesis in MCL by a direct paracrine effect on endothelial cells.
SOX11-positive primary MCL showed a significant enrichment in angiogenic-related expression signatures and higher MVD than negative tumors. In addition, PDGFA was strongly expressed in SOX11-positive MCL but not detected in negative tumors, strongly supporting that the SOX11-PDGFA–related angiogenic development identified in the experimental models also plays a role in human tumors. SOX11-negative MCL usually present clinically with non-nodal leukemic disease whereas SOX11-positive tumors involve lymph nodes and have extensive extranodal infiltration.4,5 The SOX11-dependent “angiogenic switch” discovered in this study may explain MCL clinical heterogeneity because the low angiogenic potential of SOX11-negative cells may impair their tissue infiltration and retain them in the bloodstream whereas SOX11-induced angiogenesis facilitates tissue infiltration and growth of the SOX11-expressing MCL cells.
The importance of tumor angiogenesis in the development and clinical progression of lymphoid neoplasias has suggested that these mechanisms may be potential targets for new therapies.43 Imatinib has proven antiangiogenic effects in a DLBCL xenograft model targeting pericytes via PDGFR-β,44 and lenalidomide impairs lymphangiogenesis in MCL preclinical models.45 The striking growth impairment of the SOX11-positive xenografts due to a significant reduction in MVD and an impaired angiogenesis, together with the direct endothelial inhibitory effect of imatinib similar to the anti-PDGFR-α in the in vitro experiments, suggest that angiogenic pathway may represent a novel therapeutic strategy for the treatment of aggressive MCL. However, imatinib treatment may have some limitations in the clinical context since the development of MCL has been observed in 2 patients during treatment of chronic myelogenous leukemia (CML) with this tyrosine kinase inhibitor.46,47 These 2 MCL cases were “blastoid” variants that had accumulated complex karyotypes including deletions of chromosome 9 (INK4a) and ATM and TP53 mutations. These genetic alterations are associated with an angiogenic switch through different mechanisms such as induction of VEGF, HIF-1, and downregulation of thrombospondin-1, respectively.48-50 Therefore, blastoid tumors may develop angiogenic stimuli alternative to the PDGFA–PDGFR-α axis.
In conclusion, we provide unique evidences on the SOX11-mediatied regulation of angiogenesis via the PDGFA pathway in MCL that in turn facilitates tumor growth. These findings in experimental models and primary human tumors support the oncogenic role of SOX11 in the aggressive behavior of MCL. Targeting the SOX11-PDGFA axis, which regulates the maintenance, migration, and proliferation of vascular endothelial cells, represents an attractive strategy to dismantle the lymphoma vasculature and therefore the growth and progression of this tumor. Modulation of this angiogenic pathway may therefore constitute a potential novel therapeutic strategy for the treatment of aggressive MCL.
The microarray data reported in this article have been deposited in the Gene Expression Omnibus database (accession numbers GSE52892, for the xenograft tumor gene expression arrays).
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Ministerio de Economía y Competitividad (BFU2009-09235, BFU2012-30857, and RYC-2006-002110 [V.A.]), the Fundació La Marató de TV3 (TV3-Cancer-I3/20130110 [V.A.]), and the Instituto de Salud Carlos III (SAF2012-38432 and PIE13/00033 [E.C.] and SAF11/30073 [M.C.C.]). Fondo Europeo de Desarrollo Regional. Unión Europea. Una manera de hacer Europa.
Authorship
Contribution: J.P. performed all of the in vitro and in vivo experiments, and quantified MVD in both xenograft and MCL primary tumors; M.C.V. performed ChIP experiments; M.L.R. performed WB experiments; A.E. performed luciferase assays; G.C. performed statistical analysis; E.P.-R. performed angiogenic experiments in vitro; P.J. performed GEP analysis; I.R.-C. performed immunohistochemistry experiments and quantified MVD in MCL primary tumors; M.C.C. supervised the angiogenic experiments in vitro; E.C. identified morphologically MCL tumors, analyzed data, and supervised experiments; V.A. designed, performed, and supervised experiments, analyzed data, and wrote the manuscript; and all authors discussed the results and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Virginia Amador, Centre Esther Koplowitz (CEK), C/Rosselló 153, Barcelona-08036, Spain; e-mail: vamador@clinic.ub.es.

![Figure 2. SOX11 promotes endothelial tube formation, cell proliferation and migration, and activation of angiogenic signaling cascades in vitro. (A) Top panel, Representative images showing the capillary tube formation by HUVECs growing on Matrigel matrix for 6 hours in the presence of control RPMI + 10% FBS, SOX11-positive or SOX11-negative CM on Matrigel matrix. Bottom panel, Quantification of the number of branching points representing tube formation by HUVECs cultured for 6 hours in RPMI + 10% FBS or SOX11-positive and SOX11-negative CM from Z138, JEKO1, and GRANTA519 MCL cell lines. SOX11-positive CM significantly promoted a prominent increase in tube formation compared with CM from SOX11-negative cells (126 vs 89 branching points, P = .0002, 121 vs 100 branching points, P = .009, and 102 vs 70 branching points, P < 1 × 10−4 in Z138, JEKO1, and GRANTA519) and RPMI + 10% FBS (126 vs 76 branching points, P < 1 × 10−4, 121 vs 76 branching points, P < 1 × 10−4, and 102 vs 76 branching points, P < 1 × 10−4 in Z138, JEKO1, and GRANTA519). Conversely, SOX11-negative CM did not have an effect on tube formation compared with RPMI + 10% FBS (89 vs 76 branching points, P = .2, 70 vs 76 branching points, P = .3 in Z138 and GRANTA519). (B) Kinetics of HUVEC growth in SOX11-positive CM (blue) or SOX11-negative CM (yellow) derived from SOX11-positive and SOX11-silenced Z138 (solid line) and JEKO1 (dashed line) MCL cell lines. Graph showing the optical density (620 nm) after staining the HUVECs with 0.2% crystal violet and measured at 620-nm wavelengths at the indicated time points (1, 3, and 5 days). (C) Graph displaying the percentage of migratory HUVEC cells toward SOX11-positive and SOX11-negative CM from Z138, JEKO1, and GRANTA519 MCL cell lines, relative to the percentage of migratory HUVEC cells toward RPMI + 10% FBS. HUVECs displayed significantly increased migration toward SOX11-positive CM cell lines (±90% migration) compared with SOX11-negative CM (±30%-70% migration, in Z138 and GRANTA519 [P < 1 × 10−4] and JEKO1 [P = .009]). (D) Left panel, WB experiments showing expression levels of basal and phosphorylated forms of FAK, Akt, and Erk1/2 proteins in HUVECs cultured in RPMI + 10% FBS or SOX11-positive and SOX11-negative CM from Z138 and GRANTA519 MCL cell lines for 3 hours. GAPDH was used as a loading control. Right panel, Fold change differences of phosphorylated forms of FAK, Akt, and Erk1/2 proteins in HUVECs cultured in RPMI + 10% FBS or SOX11-positive and SOX11-negative CM from Z138 and GRANTA519 MCL cell lines for 3 hours, correlated by quantification of GAPDH expression levels. Bar plot represents the mean percentage ± SD, n = 3. P-val is shown. The significance of difference was determined by independent samples Student t test. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/14/10.1182_blood-2014-04-569566/4/m_2235f2.jpeg?Expires=1767759156&Signature=YbxSwu5ZyMw~moB8nq9vZrgk7tqckUGMYcUf~Gdi~RfyJLqNnWJwXqIg7OOATYXzcF6cf3IOpUNgW-PD7LbKYyAtx-hXaqNwYMoNgMH006cddZt3YPBo2MjCcZcOKv5DT98dgXWe2Pwz0xtzg7ldHXsFo2iLa3nJC3SWLfX5WVEIDOluvhnhVxyLGdRr-x2vNSIzQVzheMQ5LJ346e880tXCMlyShTLkARoG~-yWYyGR0GNrbHgB~WYmTAhQz2JHsMSSh~u30xhZTfNus4Zt-po-LBUaBxy89GkgG75SrqRCjKlL3~-mDsELg9FVYUCjtpuP87S-7yfdSVMsGQHAtg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)