Key Points
Haploinsufficiency of Sox18 reveals an important role for VEGFD in regulating blood vascular development in vivo in vertebrates.
VEGFD acts through mitogen-activated protein kinase kinase–extracellular signal-regulated kinase to modulate the activity and nuclear concentration of endothelial-specific transcription factor SOX18.
Vascular endothelial growth factor-D (VEGFD) is a potent pro-lymphangiogenic molecule during tumor growth and is considered a key therapeutic target to modulate metastasis. Despite roles in pathological neo-lymphangiogenesis, the characterization of an endogenous role for VEGFD in vascular development has remained elusive. Here, we used zebrafish to assay for genetic interactions between the Vegf/Vegf-receptor pathway and SoxF transcription factors and identified a specific interaction between Vegfd and Sox18. Double knockdown zebrafish embryos for Sox18/Vegfd and Sox7/Vegfd exhibit defects in arteriovenous differentiation. Supporting this observation, we found that Sox18/Vegfd double but not single knockout mice displayed dramatic vascular development defects. We find that VEGFD–mitogen-activated protein kinase kinase–extracellular signal-regulated kinase signaling modulates SOX18-mediated transcription, functioning at least in part by enhancing nuclear concentration and transcriptional activity in vascular endothelial cells. This work suggests that VEGFD-mediated pathologies include or involve an underlying dysregulation of SOXF-mediated transcriptional networks.
Introduction
Vascular endothelial growth factor (VEGF) signaling pathways regulate diverse processes during vascular development.1,-3 In mammals, the VEGF family of growth factors3 bind to and activate 3 main receptors, VEGFR1, VEGFR2, and VEGFR3. Among these growth factors, VEGFA controls both vasculogenesis and angiogenesis.1,4,5 Mouse embryos lacking a single Vegfa allele are embryonic lethal because of severe blood vascular defects1,2 ; in zebrafish, knock down of Vegfa signaling leads to a block in arteriovenous differentiation and angiogenesis.5,6 VEGFB plays a role in cardiac and coronary development,7 whereas VEGFC controls the earliest lymphatic endothelial cell migration in the embryo during lymphangiogenesis.8
The function of VEGFD during embryonic vascular development remains to be clarified. VEGFD is capable of binding to and activating signaling through a number of VEGF receptors,9,10 and upregulation of VEGFD promotes pathological neo-lymphangiogenesis.11,-13 In addition, VEGFD overexpression affects blood vascular angiogenesis.14,15 Despite these capabilities, Vegfd-null mice display no obvious vascular defects16 and Vegfd/Vegfc double mutant mice are indistinguishable from Vegfc mutants, suggesting that VEGFD does not influence vascular development.17
Vasculogenesis and angiogenesis are also tightly regulated by key transcription factors in vivo. These include the SRY-related high mobility group domain family F (SOXF) transcription factor group. The SOXF group consists of SOX7, SOX17, and SOX18, which selectively recognize and activate transcription from a common consensus sequence.18 In vertebrates, all of the SOXFs are expressed in the developing blood vasculature and have been found to regulate vascular developmental processes in a redundant manner.19,,,-23 Of the SOXF factors, SOX18 primarily regulates lymphatic fate in a subpopulation of venous endothelial cells, whereas SOX7 and SOX17 act as modifiers of SOX18 function in some genetic backgrounds in mice.23 In zebrafish, during early blood vascular development, Sox18 and Sox7 redundantly regulate arteriovenous specification.19,-21 In humans, heterozygous nonsense mutations within the transactivation domain of SOX18 lead to hypotrichosis-lymphedema-telangiectasia syndrome, associated with variable vascular defects.24
In the present study, we used zebrafish to probe for genetic interactions between SoxFs and Vegfs. We identified a severe blood vascular phenotype during development with combined Sox18/Vegfd loss of function in zebrafish. We also observed this interaction in double knockout mice. We showed that VEGFD is capable of enhancing the nuclear distribution and activity of SOX18 in vitro and of modulating SoxF-dependent transcriptional activation in vivo via mitogen-activated protein kinase kinase–extracellular signal-regulated kinase (MEK-ERK) signaling. Our results identify the first endogenous role for VEGFD during blood vascular development, acting through the modulation of SOX18 activity. This conserved interaction is critical for the formation of a functional blood vascular system in the developing embryo.
Materials and methods
Mouse and zebrafish
Vegfd−/− mice have been previously described.16 Sox18−/− mice on a mixed 129/CD1 genetic background were generated and genotyped as previously described.23 To generate Sox18/Vegfd double knockout mice, we crossed Sox18−/− females (129/CD1, mixed background) with Vegfd-nullizygous males (C57BL/6 background). Sox18+/−, Vegfd+/− females were then back-crossed with Sox18−/− males. In the second generation, Sox18+/−, Vegfd+/− females and Sox18+/−, Vegfd-/Y males were bred together to generate double knockout embryos. The breeding strategy was designed this way because Vegfd is located on the X chromosome.
Morpholino oligomers, mRNA injections, and chemical treatments
The vegfd start codon (ATG) and splice-site (spl) targeting morpholino oligomers (MOs) (Genetools, LLC) (supplemental Table 3, available on the Blood Web site) were injected at a concentration of 5 ng or 10 ng/embryo. The specificity of the spl MO (MO spl-vegfd) was evaluated by performing quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) to assess messenger RNA (mRNA) expression levels in embryos injected with different amounts of MO (5 and 10 ng). vegfd mRNA for rescue experiments was made as previously described.29 Previously published and validated MOs include MO-sox18,19 MO-sox7,19 MO-vegfc,30 MO-flt4,29 MO-kdr,31 and MO-kdrl32 were injected at a concentration of 5 or 10 ng/embryo.
To inhibit VEGFR tyrosine kinase activity, SU5416 was used as previously described (SU5416; Sigma Aldrich).33 To study signaling downstream of Vegfd, we used VEGFR3 inhibitor (MAZ51; Merck), PI3-kinase inhibitors (Wortmannin; Sigma-Aldrich and LY294002; Sigma-Aldrich),34 and MEK inhibitors (U0126; Sigma-Aldrich and PD98059; Cell Signaling).34,35
Immunohistochemistry and in situ hybridization
Zebrafish embryos for sectional analysis were fixed overnight with 4% paraformaldehyde at 4°C. The embryos were then manually processed through an ethanol and xylene series before being embedded in paraffin. Paraffin-embedded embryos were sectioned using a Leica microtome into 7-μm sections. Nuclei were visualized by using 4′,6-diamidino-2-phenylindole (DAPI). Whole-mount in situ hybridization of zebrafish embryos was performed as previously described.36 Probes used in this study were: ephrinB2a,37 dab2,38 kdrl,39 gfp, etv2,40 and vegfd.25 (Additional methods can be found in supplemental Methods.)
Results
Sox7/Vegfd or Sox18/Vegfd double knockdown embryos display arteriovenous fusion defects in zebrafish
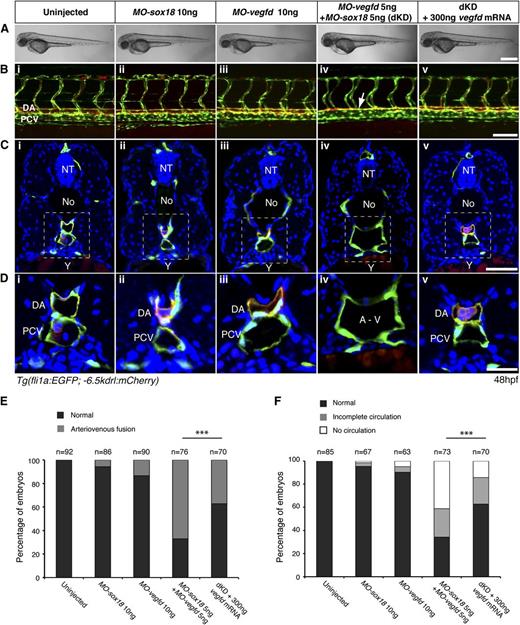
To screen for genetic interactions between SoxF transcription factors and the Vegf signaling pathway, we used zebrafish knockdown approaches. We injected previously validated19 MOs against sox18 or sox7 together with MOs targeting kdr, kdrl, flt4, vegfc, and vegfd (Table 1). We did not knock down Vegfa (a/b), Plc-ϒ, or Sox17 because in these cases early phenotypes have been reported that would be epistatic to specific later vascular defects in double loss-of-function animals.5,41,42 We examined the overall gross phenotype and the developing vasculature of injected embryos at 48 hours post fertilization (hpf) (Figure 1). At this stage, the dorsal aorta (DA) and posterior cardinal vein (PCV) have formed distinct vessels in control embryos (Figure 1Bi-iii and Table 1) and arteriovenous separation can be clearly visualized in transverse sections through the trunk (Figure 1C and Di-iii).
Sox18/Vegfd double loss-of-function causes arteriovenous fusion in zebrafish embryos. (A) Lateral views of gross morphology of zebrafish embryos at 48 hpf. (B-D) At 48 hpf, DA and PCV are formed in uninjected and single morpholino–injected control embryos (MO-sox18 ATG, MO-vegfd ATG) (i-iii). dKD embryos displayed DA and PCV fusion as shown on lateral view (Biv, white arrow) and transverse section (C,Div). Double morphants were rescued by injection of vegfd mRNA (v). Dashed lines in panel C indicate the magnified area in panel D. (E-F) Quantitative data show the number of embryos with arteriovenous and circulation defects upon MO injection. ***P < .001 by 1-way analysis of variance (ANOVA) test. No, notochord; NT, neural tube; Y, yolk. Scale bars represent 200 μm (A), 50 μm (B-C), and 20 μm (D).
Sox18/Vegfd double loss-of-function causes arteriovenous fusion in zebrafish embryos. (A) Lateral views of gross morphology of zebrafish embryos at 48 hpf. (B-D) At 48 hpf, DA and PCV are formed in uninjected and single morpholino–injected control embryos (MO-sox18 ATG, MO-vegfd ATG) (i-iii). dKD embryos displayed DA and PCV fusion as shown on lateral view (Biv, white arrow) and transverse section (C,Div). Double morphants were rescued by injection of vegfd mRNA (v). Dashed lines in panel C indicate the magnified area in panel D. (E-F) Quantitative data show the number of embryos with arteriovenous and circulation defects upon MO injection. ***P < .001 by 1-way analysis of variance (ANOVA) test. No, notochord; NT, neural tube; Y, yolk. Scale bars represent 200 μm (A), 50 μm (B-C), and 20 μm (D).
Strikingly, double knockdown (dKD) of Sox18/Vegfd or Sox7/Vegfd, but no other combination, led to arteriovenous fusion and loss of blood circulation in the posterior region of the embryo by 48 hpf (Figure 1B-Div; Table 1; supplemental Figures 1 and 2). This phenotype was indistinguishable from that of Sox18/Sox7 dKD-injected embryos, which has been previously reported.19,-21 Early migration of angioblast populations43 appeared normal at 16 hpf by etv2 whole-mount in situ hybridization (supplemental Figure 3), suggesting that vasculogenesis initiated normally in these animals but progressed to arteriovenous fusion at later stages. We controlled for the specificity and efficacy of our vegfd MOs by targeting 2 separate MOs against both the start codon and a splice site in the vegfd pre-mRNA and by using qRT-PCR to validate the splice MO (supplemental Figure 4). We also injected vegfd mRNA in a series of rescue assays (Figure 1A-Dv). The phenotypic penetrance of ∼65% of the dKD embryos was rescued to ∼35% when vegfd mRNA was introduced (Figure 1E-F). These results indicate that in the absence of one individual SoxF (Sox7 or Sox18), the combined activity of the remaining compensatory SoxF as well as Vegfd is required for normal arteriovenous development in zebrafish.
Sox18 and Vegfd genetically interact to control blood vascular development in mice
Given the surprisingly selective interaction observed in zebrafish, we generated Sox18/Vegfd double knockout mice to confirm the relevance of this interaction in a mammalian system. Because both Sox7 and Sox17 knockout mice display early embryonic lethal phenotypes,44 we focused on Sox18/Vegfd double knockout animals. Previous studies have shown that Sox18 knockout embryos exhibit only a mild coat phenotype on a mixed 129/CD1 genetic background.45 In contrast, Sox18 knockout embryos on a pure C57BL/6 background display a severe lymphatic vascular phenotype with subcutaneous edema, and die in utero around 14.5 days post coitum (dpc).23,46
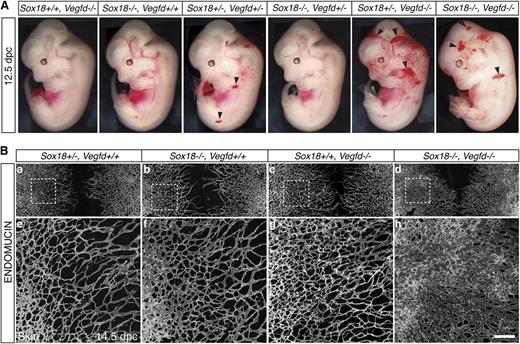
We generated an allelic series of Sox18/Vegfd double knockout embryos at a variety of time points. Phenotypes of embryos were assessed at several developmental stages (supplemental Table 1). Combinations of heterozygous and homozygous double mutants led to dramatic subcutaneous hemorrhaging (Figure 2A; supplemental Table 1). The phenotype was present at 12.5 dpc throughout the body (black arrowheads), which we considered suggestive of an underlying blood vascular defect. We observed this phenotype from as early as 11.5 dpc (supplemental Figure 5A, black arrow), although the most severe phenotypes were seen around 13.5 dpc, characterized by generalized hemorrhage and edema (supplemental Figure 5A, white arrowhead) and led to embryonic lethality by 16.5 dpc. In this mixed genetic background (129/CD1/C57BL/6), we observed a gene dosage–dependent penetrance of the phenotype ranging from 35% to 51% (a total of 198 embryos were analyzed; details of phenotypes and penetrance are given in supplementa1 Table 1 and supplemental Figure 5B).
Vascular phenotypes of Sox18/Vegfd double knockout mouse embryos. (A) Severity of the blood vascular phenotype of 12.5 dpc double mutant embryos in allelic series. Blood vascular phenotypes were increased depending on gene dosage loss of function. Blood vascular defects are characterized by generalized subcutaneous hemorrhage (black arrowheads). (B) Increased density of the blood vascular network in double knockout mice. Whole-mount immunofluorescence with the pan-endothelial cell marker ENDOMUCIN on embryonic skin at 14.5 dpc (a-d). At this stage, the blood vasculature in this area has developed and migrated toward the midline of the embryo. (e-h) Sox18/Vegfd double knock out displayed skin with increased vessel density (h) compared with Sox18+/−, Vegfd+/+ control embryos (e) and single knockout embryos (f-g). Dashed lines in panels a-d indicate the magnified area in panels e-h. Scale bar represents 400 μm.
Vascular phenotypes of Sox18/Vegfd double knockout mouse embryos. (A) Severity of the blood vascular phenotype of 12.5 dpc double mutant embryos in allelic series. Blood vascular phenotypes were increased depending on gene dosage loss of function. Blood vascular defects are characterized by generalized subcutaneous hemorrhage (black arrowheads). (B) Increased density of the blood vascular network in double knockout mice. Whole-mount immunofluorescence with the pan-endothelial cell marker ENDOMUCIN on embryonic skin at 14.5 dpc (a-d). At this stage, the blood vasculature in this area has developed and migrated toward the midline of the embryo. (e-h) Sox18/Vegfd double knock out displayed skin with increased vessel density (h) compared with Sox18+/−, Vegfd+/+ control embryos (e) and single knockout embryos (f-g). Dashed lines in panels a-d indicate the magnified area in panels e-h. Scale bar represents 400 μm.
To determine whether hemorrhaging was associated with vascular defects, we examined the subcutaneous blood vascular network in 14.5 dpc embryonic skin by whole-mount immunofluorescence staining for the pan-endothelial cell marker ENDOMUCIN. The subcutaneous blood vascular network grows progressively from the lateral aspect of the embryo to the dorsal midline, where vessels from 2 distinct migration fronts fuse.47 Analysis of the dissected skin of Sox18/Vegfd double knockout mice revealed that, although angiogenesis had given rise to a network of subcutaneous blood vessels, these vessels were dilated in cross-section (supplemental Figure 5Cii, arrows) and displayed significantly increased density (Figure 2B; supplemental Figure 5D) compared with wild-type vessels. These data suggest that dramatic blood vascular defects are responsible for the surface hemorrhage observed in double knockout embryos.
Sox18/Vegfd double knockout mouse embryos display dilation of the major vessels
We assessed arterial and venous morphology in Sox18/Vegfd double knockout mouse embryos. Transverse sections of 11.5 dpc embryonic trunks were stained with specific vascular markers NEUROPILIN-2, CAVEOLIN, and CD34 to highlight the DA and cardinal veins (CVs) (Figure 3A). We observed both dilated DAs and CVs in the double knockout embryos compared with control embryos (Sox18+/−, Vegfd+/+) (Figure 3A, n = 2). In addition, we performed whole-mount immunofluorescence staining for VEGFR3 (green) and PECAM (red). Three-dimensional optical projection tomography was used to examine the whole embryonic vasculature (Figure 3Bi,iii; supplemental movies 1-2). Double knockout embryos displayed a dilated left, right, and midline DA when compared with control embryos (Sox18+/−, Vegfd+/+) (n = 3) (Figure 3Bii,iv; white arrowhead). Interestingly, we also noticed 1 arterial branch missing in double knockout embryos (Figure 3Biv, asterisk). These data showed that the defects occur early in development. This genetic interaction implies that SOX18-positive endothelium should neighbor a source of VEGFD during blood vascular development. Hence, we evaluated Vegfd and Sox18 expression during vascular formation using in situ hybridization. Vegfd expression was detected adjacent to the DA and CV, which expressed Sox18 in mouse (11.5 dpc) and zebrafish (24 hpf) (Figure 3C-D).23,46,48
Sox18/Vegfd double knockout mouse embryos display defects of the major vessels. (A) Immunofluorescence staining on transverse section of 11.5 dpc embryo using NEUROPILIN-2 (green), CAVEOLIN (red), and CD34 (blue) revealed the dilation of the DA and CV in the double knockout embryo compared with the control. (B) Frontal view of 11.5 dpc embryos whole mount stained with PECAM (red) and VEGFR3 (green) (i,iii). Rendering of the major vasculature using IMARIS software (i-iv), showing the dilated left, right, and midline DA in the double knockout embryo (iv) compared with control embryos Sox18+/−, Vegfd+/+ (ii, white arrowhead). Asterisk indicates the missing interarterial branching in double knockout embryo. (C) In situ hybridization shows both the expression profile of Sox18 and Vegfd on transverse sections of mouse embryo at 11.5 dpc. Sox18 mRNA was detected in the endothelium of both DA and CV at 11.5 dpc (red). Vegfd mRNA was detected in the vicinity of the DA and CV (purple). (D) In situ hybridization shows the expression profile of vegfd in zebrafish at 24 hpf. (i,iii) vegfd mRNA was detected at the tailbud (i, black arrow), neurons (iii, black arrows), and trunk (iii, bracket). (ii,iv) Sense probe was used as a negative control for the specificity of vegfd signal in in situ. Dashed lines (i,ii) indicate the magnified area in (iii-iv). A, anterior; CSG, cervical sympathetic ganglia; D, dorsal; H, heart; L, lateral; M, medial. Scale bar represents 200 μm (A), 100 μm (C), 200 μm (Dii), and 100 μm (Div).
Sox18/Vegfd double knockout mouse embryos display defects of the major vessels. (A) Immunofluorescence staining on transverse section of 11.5 dpc embryo using NEUROPILIN-2 (green), CAVEOLIN (red), and CD34 (blue) revealed the dilation of the DA and CV in the double knockout embryo compared with the control. (B) Frontal view of 11.5 dpc embryos whole mount stained with PECAM (red) and VEGFR3 (green) (i,iii). Rendering of the major vasculature using IMARIS software (i-iv), showing the dilated left, right, and midline DA in the double knockout embryo (iv) compared with control embryos Sox18+/−, Vegfd+/+ (ii, white arrowhead). Asterisk indicates the missing interarterial branching in double knockout embryo. (C) In situ hybridization shows both the expression profile of Sox18 and Vegfd on transverse sections of mouse embryo at 11.5 dpc. Sox18 mRNA was detected in the endothelium of both DA and CV at 11.5 dpc (red). Vegfd mRNA was detected in the vicinity of the DA and CV (purple). (D) In situ hybridization shows the expression profile of vegfd in zebrafish at 24 hpf. (i,iii) vegfd mRNA was detected at the tailbud (i, black arrow), neurons (iii, black arrows), and trunk (iii, bracket). (ii,iv) Sense probe was used as a negative control for the specificity of vegfd signal in in situ. Dashed lines (i,ii) indicate the magnified area in (iii-iv). A, anterior; CSG, cervical sympathetic ganglia; D, dorsal; H, heart; L, lateral; M, medial. Scale bar represents 200 μm (A), 100 μm (C), 200 μm (Dii), and 100 μm (Div).
Having observed early defects in mouse embryonic arteries and veins, we further characterized the arteriovenous defects observed in dKD zebrafish. We evaluated the expression of the arterial marker, ephrinB2a, and the venous marker, dab2, in 26 hpf embryos. dKD Sox18/Vegfd embryos showed ectopic expression of arterial marker ephrinB2a in the vein and of venous marker dab2 in the artery (supplemental Figure 6). Notably, shunt formation between the artery and vein in Sox18/Vegfd dKD embryos was observed (supplemental Figure 6). Although this is a distinct phenotype from that observed in mouse embryos, these defects in arteriovenous differentiation are consistent with a conserved early blood vascular defect.
SOX18-driven transcription and SOX18 nuclear distribution is enhanced by VEGFD treatment in vitro in endothelial cells
To investigate a possible role for VEGFD in regulating SOX18 transcriptional activity, we treated a lymphatic endothelial cell line49 with VEGFD at 200 ng/mL and evaluated SOX18 transcriptional activity. First, we performed luciferase assays in these cells using a vector containing 1889 bp of the proximal mouse VCAM1 promoter construct (pGL2B, VC1889), previously identified as a direct target of SOX18.50 The VCAM1-driven luciferase reporter gene had basal levels of expression posttransfection that was likely from endogenous levels of other SOXF transcription factors. Importantly, this promoter activity was significantly enhanced approximately 2-fold in transfected cells treated with VEGFD (Figure 4A, n = 3, P < .01). The enhanced activity of this promoter fragment was suppressed when the cells were co-transfected with constructs driving the expression of the Ragged Opossum (Sox18RaOp) form of Sox18, which encodes a dominant-negative protein that suppresses the endogenous function of all SOXF family transcription factors (Figure 4A).48 These assays indicate that gain of VEGFD function can modulate SOX18 target gene expression in a SOX18-dependent manner in vitro.
VEGFD modulates SOXF activity in vitro. (A) Luciferase assay to evaluate the effect of VEGFD on the induction of VCAM1, a SOX18 direct gene target. Transfection of lymphatic endothelial cells (LECs) with VC1889 vector in combination with VEGFD treatment leads to induction of luciferase reporter expression, whereas co-transfection of LECs with VC1889 and RaOp (a pSG5 constructs driving the expression of a dominant negative Sox18 mutant) leads to suppression of luciferase activity. (B-D) Expression levels of SOX18 target genes (Prox1, Mmp7, Tie1) (qRT-PCR) in LECs were upregulated by VEGFD treatment (200 ng/mL), and induction was suppressed by transfection with RaOp expression vector. (E) Western blot using nuclear and cytoplasmic extracts from LECs. SOX18 protein levels were elevated in the nucleus after 1 hour of VEGFC or VEGFD stimulation. (F) qRT-PCR and (G) luciferase assay using Sox18 promoter cloned into pGL2B luciferase reporter construct showed that Sox18 transcription was not altered after treatment of LECs with VEGFs. (H) Immunofluorescence staining on LECs for endogenous SOX18 (green), PECAM (red), and DAPI (blue) indicated that VEGFs triggers nuclear condensation of SOX18 (asterisk). Insets show magnifications of area in dashed lines. (I) Immunofluorescence staining on human umbilical vein endothelial cells (HUVECs) for endogenous SOX18 (red), Phalloidin (green), and DAPI (blue). VEGFD treatment triggers nuclear condensation of SOX18 (asterisk). Scale bar represents 50 μm. (J) Intensity scan profile showing intensity values for SOX18 (red) and DAPI (blue), measured across a cell nucleus. The position of the scan line is shown (I, dashed line). (K) Percentage of cells with increased SOX18 in nucleus (approximately 100 cells counted per field of view over at least 3 different fields of view). Cyto., cytoplasm; Nuc., nucleus. The data are shown as the mean ± standard error of the mean of 3 independent experiments. **P < .01, *P < .05 by 1-way ANOVA test.
VEGFD modulates SOXF activity in vitro. (A) Luciferase assay to evaluate the effect of VEGFD on the induction of VCAM1, a SOX18 direct gene target. Transfection of lymphatic endothelial cells (LECs) with VC1889 vector in combination with VEGFD treatment leads to induction of luciferase reporter expression, whereas co-transfection of LECs with VC1889 and RaOp (a pSG5 constructs driving the expression of a dominant negative Sox18 mutant) leads to suppression of luciferase activity. (B-D) Expression levels of SOX18 target genes (Prox1, Mmp7, Tie1) (qRT-PCR) in LECs were upregulated by VEGFD treatment (200 ng/mL), and induction was suppressed by transfection with RaOp expression vector. (E) Western blot using nuclear and cytoplasmic extracts from LECs. SOX18 protein levels were elevated in the nucleus after 1 hour of VEGFC or VEGFD stimulation. (F) qRT-PCR and (G) luciferase assay using Sox18 promoter cloned into pGL2B luciferase reporter construct showed that Sox18 transcription was not altered after treatment of LECs with VEGFs. (H) Immunofluorescence staining on LECs for endogenous SOX18 (green), PECAM (red), and DAPI (blue) indicated that VEGFs triggers nuclear condensation of SOX18 (asterisk). Insets show magnifications of area in dashed lines. (I) Immunofluorescence staining on human umbilical vein endothelial cells (HUVECs) for endogenous SOX18 (red), Phalloidin (green), and DAPI (blue). VEGFD treatment triggers nuclear condensation of SOX18 (asterisk). Scale bar represents 50 μm. (J) Intensity scan profile showing intensity values for SOX18 (red) and DAPI (blue), measured across a cell nucleus. The position of the scan line is shown (I, dashed line). (K) Percentage of cells with increased SOX18 in nucleus (approximately 100 cells counted per field of view over at least 3 different fields of view). Cyto., cytoplasm; Nuc., nucleus. The data are shown as the mean ± standard error of the mean of 3 independent experiments. **P < .01, *P < .05 by 1-way ANOVA test.
To strengthen this observation, we evaluated the expression of other SOX18 endogenous target genes in these cells. We examined the levels of known targets Prox1,46 Mmp7,51,52 and Tie1 (unpublished data). The endogenous expression of these SOX18 target genes was upregulated approximately twofold in the presence of VEGFD and in all cases this was suppressed in the presence of SOX18RaOp dominant-negative protein (Figure 4B-D, n = 3, P < .05).
We next examined SOX18 protein subcellular localization in cells treated with VEGF ligands. Using VEGFC and VEGFD treatments, we found that the level of SOX18 protein was elevated in the nucleus by western blot analysis of nuclear protein isolated after stimulation for 1 hour (Figure 4E). Importantly, this regulation was independent of Sox18 transcription based on both qRT-PCR and Sox18 promoter-luciferase assays (Figure 4F-G). We treated cells with VEGFD, VEGFC, and VEGFA (200 ng/mL) for 1 hour and then examined SOX18 protein distribution. We found that although SOX18 is detectable in the nucleus of lymphatic endothelial cells without treatment, SOX18 distribution within the nucleus was significantly more widespread following all treatments (Figure 4H, asterisks). We also examined these effects in an alternative endothelial cell culture model-HUVECs (Figure 4I, asterisks). We treated HUVECs with VEGFD (300 ng/mL) and quantified SOX18 levels in the nucleus by ImageJ analysis of immunofluorescence localization. Fluorescence intensity for SOX18 analyzed in cytoplasm relative to nuclei confirmed that VEGFD promotes higher localization of this transcription factor in nuclei (Figure 4J-K).
This approach assays only capability in vitro and not necessity in vivo of the identified interactions. Hence, these observations in cultured endothelial cells identify a biochemical capability of the VEGFs to modulate the nuclear concentration and activity of SOX18.
SoxF-driven gene expression is modulated by Vegfd in vivo in zebrafish
To determine whether this capability of VEGFD is relevant in an in vivo setting, we took advantage of a transgenic zebrafish line, Tg(−6.5kdrl:EGFP), in which EGFP is driven by a 6.5-kb kdrl promoter.28 Previous studies have noted that the expression of this transgene is reduced in Sox18/Sox7 dKD-injected embryos.20,21
We investigated this further in a Tg(−6.5kdrl:EGFP;fli1a.ep:DsRedEx) double transgenic embryos using confocal microscopy, in situ hybridization and real-time PCR analysis (Figure 5A-D). Zebrafish embryos with single knock down of either Sox18 or Sox7 displayed a mild reduction in enhanced green fluorescent protein (EGFP) expression as compared with uninjected control embryos (Figure 5A-B). In contrast, dKD Sox18/Sox7 embryos display a near-complete loss of EGFP expression at the level of both transcript and protein, whereas the fli1a.ep:DsRedEx transgene remained unchanged, the latter of which indicated the presence of endothelium (Figure 5A-B,D). To determine if this reflects an essential requirement of Sox18/Sox7 for kdrl transcription, we evaluated the endogenous kdrl transcript in dKD embryos. Although EGFP transcript levels were significantly reduced in dKD embryos, the difference in endogenous kdrl transcript between control and Sox18/Sox7 dKD was subtle compared with the reduction in EGFP expression (Figure 5C, n = 6, P < .05; supplemental Figure 7). This indicates that the kdrl:EGFP transgenic line is a more sensitive reporter of SoxF-driven gene expression than the endogenous kdrl locus.
Vegfd modulates SoxF driven gene expression in vivo in zebrafish. (A) Confocal projection of Tg(−6.5kdrl:EGFP; fli1a.ep:DsRedEx), in which the EGFP transgene is driven by a 6.5-kb kdrl promoter fragment. The expression of EGFP but not DsRed was selectively reduced in single morphants and appeared absent in Sox18/Sox7 dKD embryos. (B) In situ hybridization for EGFP transcript confirmed downregulation in Sox18/Sox7 dKD. (C-D) qRT-PCR showed a mild reduction of endogenous kdrl mRNA concentration (C) in dKD embryos, whereas EGFP expression levels were dramatically decreased (D) (relative to cdh5). (E) Map of the 6.5-kb kdrl promoter fragment showing the position of SOX responsive elements (S1, S2) within the −4.3 to −3.5 kb minimal promoter region previously identified as sufficient to drive EGFP expression.53 The 6.3-kb Δsox promoter fragment was generated by excising approximately 200 bp that includes S1 and S2. Δkdrl promoter was generated by removing a 6.3-kb fragment and was used as a negative control to assess EGFP expression. (F) In confocal projection of wild-type zebrafish injected with different kdrl:EGFP constructs, the level of EGFP expression in injected embryos was scored qualitatively (G), and quantified by RT-PCR (H) (relative to cdh5). Loss of the 200-bp fragment containing S1 and S2 led to a complete loss of EGFP expression. (I) Fluorescence polarization assay showing the direct binding of SOX18 protein to the SOX response elements in vitro (S1 black and S2 gray). The 100 times excess nonlabeled wild-type DNA probes were sufficient to displace 100% of the fluorescent-tagged probes, whereas the same excess of mutated probe failed to efficiently compete (ANOVA, post hoc Dunnett's test, multiple comparison with DNA free controls, ± standard deviation, n = 3). (J) Expression level of EGFP in the transgenic line Tg(−6.5kdrl:EGFP) was assessed by qRT-PCR. Knock down of Vegfd alone influenced EGFP transcript (23% reduction). dKD of either Sox18/Vegfd or Sox7/Vegfd shows a further reduction in EGFP expression. The data are shown as the mean ± standard error of the mean of 3 to 6 independent experiments. ****P < .0001, ***P < .001, *P < .05, ns: nonsignificant by 1-way ANOVA test. Red dashed line indicates a proposed phenotypic threshold (see the “Discussion” section). Scale bars represent 100 μm (A), 200 μm (B), and 100 μm (F).
Vegfd modulates SoxF driven gene expression in vivo in zebrafish. (A) Confocal projection of Tg(−6.5kdrl:EGFP; fli1a.ep:DsRedEx), in which the EGFP transgene is driven by a 6.5-kb kdrl promoter fragment. The expression of EGFP but not DsRed was selectively reduced in single morphants and appeared absent in Sox18/Sox7 dKD embryos. (B) In situ hybridization for EGFP transcript confirmed downregulation in Sox18/Sox7 dKD. (C-D) qRT-PCR showed a mild reduction of endogenous kdrl mRNA concentration (C) in dKD embryos, whereas EGFP expression levels were dramatically decreased (D) (relative to cdh5). (E) Map of the 6.5-kb kdrl promoter fragment showing the position of SOX responsive elements (S1, S2) within the −4.3 to −3.5 kb minimal promoter region previously identified as sufficient to drive EGFP expression.53 The 6.3-kb Δsox promoter fragment was generated by excising approximately 200 bp that includes S1 and S2. Δkdrl promoter was generated by removing a 6.3-kb fragment and was used as a negative control to assess EGFP expression. (F) In confocal projection of wild-type zebrafish injected with different kdrl:EGFP constructs, the level of EGFP expression in injected embryos was scored qualitatively (G), and quantified by RT-PCR (H) (relative to cdh5). Loss of the 200-bp fragment containing S1 and S2 led to a complete loss of EGFP expression. (I) Fluorescence polarization assay showing the direct binding of SOX18 protein to the SOX response elements in vitro (S1 black and S2 gray). The 100 times excess nonlabeled wild-type DNA probes were sufficient to displace 100% of the fluorescent-tagged probes, whereas the same excess of mutated probe failed to efficiently compete (ANOVA, post hoc Dunnett's test, multiple comparison with DNA free controls, ± standard deviation, n = 3). (J) Expression level of EGFP in the transgenic line Tg(−6.5kdrl:EGFP) was assessed by qRT-PCR. Knock down of Vegfd alone influenced EGFP transcript (23% reduction). dKD of either Sox18/Vegfd or Sox7/Vegfd shows a further reduction in EGFP expression. The data are shown as the mean ± standard error of the mean of 3 to 6 independent experiments. ****P < .0001, ***P < .001, *P < .05, ns: nonsignificant by 1-way ANOVA test. Red dashed line indicates a proposed phenotypic threshold (see the “Discussion” section). Scale bars represent 100 μm (A), 200 μm (B), and 100 μm (F).
We further explored whether the kdrl promoter is a direct SoxF target in zebrafish. Earlier work performed with this transgene identified an 800-bp fragment essential to drive endothelial expression.53 In silico analysis of this 800-bp region uncovered the presence of a module made of 2 Sox-responsive elements, S1 and S2, separated by 60 nucleotides (Genomatix software) (Figure 5E). This module displays a high degree of similarity to previously published functional Sox sites.54,55 To assess the functionality of these 2 Sox sites in vivo, we excised 200 bp from the full 6.5-kb kdrl promoter, containing the 2 putative Sox sites by restriction digest. DNA was injected to analyze transient transgene expression of the 6.5-kb promoter or a 6.3-kb Δsox promoter. Excision of the 200-bp fragment led to strong reduction of EGFP expression post-DNA injection (Figure 5F-H). To show that these putative Sox-responsive elements are bound by SOX18 protein, fluorescence polarization experiments were used.56 We found that SOX18 has the capability to bind directly to both of the sites present in this 200-bp region (Figure 5I; supplemental Figure 8). This result suggests that kdrl is probably directly modulated by SoxF transcription factors in zebrafish and serves as an in vivo readout of SoxF transcriptional activity.
We took advantage of this observation and knocked down Vegfd in combination with either Sox18 or Sox7 in the Tg(−6.5kdrl:EGFP) line. We used qRT-PCR to quantify EGFP mRNA expression levels relative to the endogenous levels of cdh5 (VE-Cadherin). Embryos carrying a single copy of the kdrl:EGFP transgene (heterozygotes) with Vegfd knockdown displayed a mild reduction in EGFP expression levels compared with control uninjected embryos. Single knock down of Sox7 but not Sox18 led to mild reductions in EGFP transcript levels, whereas the dKD of Vegfd with either Sox7 or Sox18 led to further reduced EGFP transcript levels (Figure 5J, n = 6, P < .001; supplemental Figure 9). Taken together with the previous findings, these data suggest that in the context of depleted Sox7 or Sox18, endogenous Vegfd modulates the transcriptional output of the remaining, compensatory SoxF in the zebrafish embryo.
VEGFD acts via MEK-ERK to modulate SOXF activity
Although VEGFD has been shown to be capable of activating signaling through several VEGFRs in different organisms,9,10 we found no evidence for genetic interactions between sox18 or sox7 and any individual vegfrs in our zebrafish double morpholino analysis (Table 1). To test which downstream signaling mechanism was involved in the interaction, we used the chemical inhibitors SU5416, MAZ51, Wortmannin, LY294002, U0126, and PD98059 that target VEGFR tyrosine kinase activity, VEGFR3, PI3K, and MEK-ERK activity. These inhibitors were tested in zebrafish together with MO-sox7 or MO-sox18 and the frequency of arteriovenous defects scored as a readout for interaction. We found that SU4516 (suboptimal concentration 0.55 μΜ) or U0126 and PD98059 (MEK inhibitors) treatment led to significant increases in the frequency of the arteriovenous fusion phenotype in combination with MO injection (Figure 6A-B; supplemental Figure 10), whereas MAZ51 (VEGFR3 blocker), Wortmannin, and LY294002 (PI3K inhibitors) did not give rise to any interaction (supplemental Table 2). Previous studies have shown that zebrafish embryos treated with SU5416 inhibitor at 1 μM display arteriovenous fusion defects, as do double kdrl/kdr MO injections.6,33 Given that SU5416 is expected to inhibit multiple VEGFRs, these data combined with the MO interactions shown in Table 1, suggest that a combination of receptors is likely to be involved. The observed interactions with U0126 and PD98059 (MEK inhibitors) treatment suggest that intracellular VEGFD signals are transduced through the MEK-ERK pathway.
Vegfd modulates SoxF transcription factor activity through the MEK-ERK signaling pathway. (A) Quantitative data showing the percentage of embryos with arteriovenous fusion phenotypes in the presence of inhibitors and MOs. Uninjected (black bars), MO-sox18 (gray bars), or MO-sox7 (white bars) injected embryos were treated with inhibitors, including tyrosine kinase inhibitor (SU5416, 0.55 μM), VEGFR3 inhibitor (MAZ51, 5 μM), PI3K inhibitors (Wortmannin, 250 nM; LY294002, 10 μM), and MEK inhibitors (U0126, 10 μM; PD98059, 15 μM). The arteriovenous fusion phenotype can be observed in combinations of MO-sox18 or MO-sox7 injection with either SU5416 inhibitor or MEK inhibitors (U0126, PD98059), but not in combination with MAZ51 or PI3K inhibitors (Wortmannin, LY294002). (B) Transverse sections of the trunk region confirmed the arteriovenous fusion phenotypes. (C) Percentage of HUVECs with SOX18 nuclear condensation after inhibitor treatments in the presence or absence of VEGFD. (D) Immunofluorescence staining on HUVECs for endogenous SOX18 (red), phalloidin (green) and DAPI (blue). VEGFD-treated cells showed nuclear condensation of SOX18 (asterisk), which was blocked by SU5416 and U0126 inhibitors, but not by MAZ51 inhibitor treatment. ***P < .001, **P < .01, *P < .05 by 1-way ANOVA test. Scale bars represent 20 μm (B) and 50 μm (D).
Vegfd modulates SoxF transcription factor activity through the MEK-ERK signaling pathway. (A) Quantitative data showing the percentage of embryos with arteriovenous fusion phenotypes in the presence of inhibitors and MOs. Uninjected (black bars), MO-sox18 (gray bars), or MO-sox7 (white bars) injected embryos were treated with inhibitors, including tyrosine kinase inhibitor (SU5416, 0.55 μM), VEGFR3 inhibitor (MAZ51, 5 μM), PI3K inhibitors (Wortmannin, 250 nM; LY294002, 10 μM), and MEK inhibitors (U0126, 10 μM; PD98059, 15 μM). The arteriovenous fusion phenotype can be observed in combinations of MO-sox18 or MO-sox7 injection with either SU5416 inhibitor or MEK inhibitors (U0126, PD98059), but not in combination with MAZ51 or PI3K inhibitors (Wortmannin, LY294002). (B) Transverse sections of the trunk region confirmed the arteriovenous fusion phenotypes. (C) Percentage of HUVECs with SOX18 nuclear condensation after inhibitor treatments in the presence or absence of VEGFD. (D) Immunofluorescence staining on HUVECs for endogenous SOX18 (red), phalloidin (green) and DAPI (blue). VEGFD-treated cells showed nuclear condensation of SOX18 (asterisk), which was blocked by SU5416 and U0126 inhibitors, but not by MAZ51 inhibitor treatment. ***P < .001, **P < .01, *P < .05 by 1-way ANOVA test. Scale bars represent 20 μm (B) and 50 μm (D).
To further validate this observation, we returned to our HUVEC model. VEGFD-induced nuclear enrichment of SOX18 was disrupted by treatment with SU5416 or MEK inhibitors (U0126) but not a VEGFR3 inhibitor (MAZ51) (Figure 6C-D). This interaction supports the in vivo findings that the MEK-ERK pathway is involved in the transduction of VEGFD-mediated signals and cooperates with SOX7 and SOX18 during vascular development.
Discussion
Here, we set out to evaluate the level of genetic interaction between 2 key pathways regulating early vascular development: SOXF transcription factors and VEGF signaling pathways. We have uncovered a previously unknown embryonic function of VEGFD during early blood vessel development. Although VEGFD has long been known through overexpression studies to be capable of regulating key developmental pathways,15 an endogenous role in the embryo has remained elusive. Surprisingly, the severe blood vascular phenotype in mice is only revealed when there is a combined loss of both VEGFD and SOX18 function. The penetrance of the double knockout mouse phenotype was around 50%, perhaps indicating the existence of further genetic modifiers or redundancy.23,57 In the zebrafish, dKD phenotypes revealed that the interaction is highly conserved and influences arteriovenous differentiation.
Both Sox7 and Sox17 knockout mice are early embryonic lethal, precluding assessment of the vasculature at stages examined here (unpublished data).44 Hence, the Sox18 knockout mice, which do not display blood vascular defects, were ideal for this study. Notably, the blood vascular phenotype in double knockout Sox18/Vegfd embryos was absent before 10.5 dpc, and we only observed the gross blood vascular phenotype from 11.5 dpc, which was severe by 12.5 dpc (Figure 2A; supplemental Figure 5A). These interactions cannot be explained by the mixed genetic background used here. Vegfd−/− mice show no phenotype on any background (or in these specific crosses from n = 23 embryos analyzed) and Sox18−/− knockout mice rarely display any blood vascular phenotype, with hemorrhaging seen in 14% of these mixed background embryos from n = 43 embryos examined here (supplemental Table 1). Relative to these phenotypic frequencies, penetrance of 55% from n = 51 double Sox18/Vegfd homozygous null embryos is highly statistically significant (Fisher's exact test P = 3.766e-05). Although this study focused on the role of this genetic interaction in blood vascular development, double loss-of-function animals in both mouse and zebrafish exhibit some lymphatic vascular phenotypes (supplemental Figure 11). However, these are likely to be secondary to earlier blood vascular defects and further studies are needed.
We show in vitro that endothelial cells treated with VEGFD, C, and A have increased SOX18 transcriptional activity. This level of activation may not reflect the level of influence seen in vivo, but does establish the biochemical potency of VEGFs to modulate SOX18 posttranscriptionally at the level of SOX18 nuclear distribution and transcriptional activity. This capability is MEK-ERK–dependent and regulates nuclear concentration and activity of SOX18. Consistently, a recent study from Deng et al35 demonstrated that increased signaling through ERK increases the nuclear detection of SOX18 and subsequently increases PROX1 expression in vivo in mice.
As a demonstration of VEGFD’s ability to influence SOX18-directed transcription in vivo, we used the Tg(−6.5kdrl:EGFP) zebrafish line. We found that −6.5kdrl:EGFP transgene expression is Sox7- and Sox18-driven. This is not a function of transgene integration position, because we also see it with independent insertions of kdrl:mCherry-CAAX (data not shown)27 and have refined potential Sox-responsive elements within the promoter (Figure 5). To a lesser extent than at this synthetic locus, endogenous kdrl gene expression levels were also modulated by Sox7 and Sox18. This in vivo tool showed that the Sox-driven EGFP expression is modified by Vegfd levels. The level of influence exerted by Vegfd at the kdrl promoter is very mild, with only a 23% reduction in vegfd morphants. Similarly, in an embryonic stem cell model of SoxF-deficient endothelial differentiation, a mild reduction of VEGFR2 mRNA expression levels (20%) has been previously observed.46 Interestingly, the modulation of kdrl may be of functional importance in the blood vessel phenotypes seen in dKD embryos given that Vegfa/Kdrl regulates arteriovenous segregation.6
The phenotypes observed in vivo in mouse were highly reminiscent of phenotypes in the dominant-negative Sox18RaOp model observed from 12.5 dpc to 14.5 dpc51 and the phenotypes seen in double MO experiments mimicked Sox7/Sox18 dKD. We propose that these results together point toward a “phenotypic threshold” model. Under this scenario, removal of 1 SoxF is being compensated for by the presence of redundant SoxF transcription factors (as shown previously19,-21,23 ) and Vegfd enhancement of the remaining SoxF activity maintains a wild-type phenotype. In the absence of Vegfd, reduced activity of the compensatory SoxF may lead to gene expression levels at a range of target loci that fall below the threshold needed to maintain normal development (indicated in Figure 5J).
Our finding of a novel capability of VEGFD to enhance SOXF transcription factor function implies that the well-documented pathological phenotypes associated with abnormal VEGFD expression may include an element of SOXF dysregulation. It will be interesting to explore whether the interactions observed here in the context of the embryo play out under pathological conditions such as cancer metastasis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the Institute for Molecular Bioscience animal house and the University of Queensland Biological Resources aquatics team for mouse and zebrafish care. Confocal microscopy was performed at the Australian Cancer Research Foundation Dynamic Imaging Centre for Cancer Biology. The authors thank Dr Stainier (Max Planck Research Institute) for the gift of the Tol2-kdrl-EGFP construct.
This work was supported by Cancer Council Queensland (APP1008392) and in part by National Health and Medical Research Council project grant (APP1048242) and fellowship (APP1011242) (M.F.); and Australian Research Council Future Fellowships (FT100100165) (B.H.), (FT110100496) (K.A.S.), and (FF0776096) (P.K.).
Authorship
Contribution: T.D. performed experiments, analyzed data, and wrote the paper; K.K., C.P.-T., L.L.G., F.F, K.A.S., V.T., and R.S. performed experiments and analyzed data; S.A.S., M.G.A., and P.K. wrote the paper; and B.M.H. and M.F. designed and performed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence. Mathias Francois, Division of Molecular Genetics and Development, Institute for Molecular Bioscience, The University of Queensland, Brisbane, Queensland 4072, Australia; e-mail: m.francois@imb.uq.edu.au.
References
Author notes
B.M.H. and M.F. contributed equally to this study.