In this issue of Blood, Zhang et al describe an exciting new small-molecule antagonist of CXCL12-CXCR4 binding with a potent ability to mobilize hematopoietic stem cells (HSCs).1 Further clinical development of this new drug, Me6TREN, may have broad applications in stem-cell mobilization for cancer and in regenerative medicine.
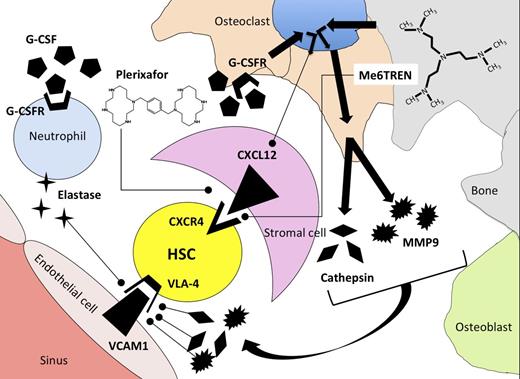
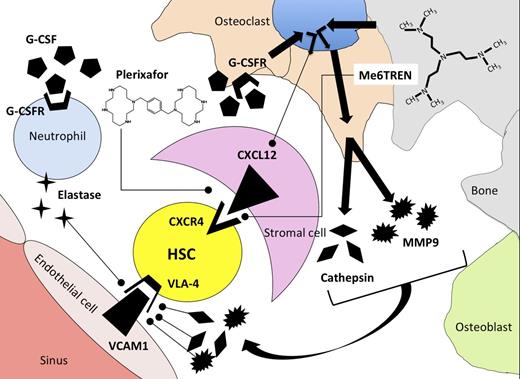
Mechanism of release of HSCs from the bone marrow microenvironment using G-CSF, plerixafor, or Me6TREN as mobilization agents. G-CSF induces synthesis of proteases elastase, cathepsin, and MMP9 by neutrophils and osteoclasts after binding to G-CSF receptors (G-CSFR). Proteases degrade adhesion molecules that tether HSCs to stromal cells in the bone-marrow microenvironment. As a secondary effect mediated through osteoclasts, G-CSF binding leads to downregulation of CXCL12 on bone-marrow stromal cells. Plerixafor antagonizes binding of CXCR4 to CXCL12, whereas Me6TREN has reported activity in antagonizing CXCR4/CXCL12 binding as well as inducing synthesis of MMP9.
Mechanism of release of HSCs from the bone marrow microenvironment using G-CSF, plerixafor, or Me6TREN as mobilization agents. G-CSF induces synthesis of proteases elastase, cathepsin, and MMP9 by neutrophils and osteoclasts after binding to G-CSF receptors (G-CSFR). Proteases degrade adhesion molecules that tether HSCs to stromal cells in the bone-marrow microenvironment. As a secondary effect mediated through osteoclasts, G-CSF binding leads to downregulation of CXCL12 on bone-marrow stromal cells. Plerixafor antagonizes binding of CXCR4 to CXCL12, whereas Me6TREN has reported activity in antagonizing CXCR4/CXCL12 binding as well as inducing synthesis of MMP9.
The field of HSC transplantation has been strongly impacted by the clinical approval of plerixafor, a CXCR4 antagonist that has been shown to be effective in mobilizing stem cells from the bone marrow into circulation in the blood.2 The clinical use of plerixafor in combination with 5 days of granulocyte colony-stimulating factor (G-CSF) administration has allowed collection of CD34+ stem cells from patients with lymphoma and myeloma undergoing autologous stem cell transplantation in fewer days and in higher numbers than had been possible when mobilizing patients with G-CSF alone.3,4 A limitation in the clinical use of plerixafor is the need to combine it with G-CSF, thus subjecting potential donors to toxicities of 2 drugs. In addition, while the addition of plerixafor to the armamentarium of drugs for stem-cell mobilization has decreased the incidence of mobilization failure from 10% to 40% to <7%,5 there are still patients in whom attempts at stem-cell collection fail, and these patients are unable to proceed to autologous stem-cell transplantation. Thus, scientists studying hematopoiesis and stem-cell transplant physicians are interested in new agents that could increase the efficiency of stem-cell mobilization and collection. To address this need, Zhang et al identified a small molecule, Me6TREN, that potently mobilized HSCs from bone marrow to peripheral blood in mice. Using flow cytometry and in vitro colony assays, they found that a single Me6TREN injection mobilized phenotypically defined lineage− sca+ c-kit+ (LSK) stem cells and hematopoietic colony-forming cells, and that the blood content of circulating progenitor cells remained elevated for 4 days after Me6TREN treatment.
The natural question, when faced with a new drug, is whether it is more effective than existing drugs. Based upon their chemical structures, it would appear that these drugs might have different mechanisms of action, because plerixafor is a 1,1′-[1,4-phenylenebis(methylene)]bis[1,4,8,11-tetraazacyclotetradecane] molecule with bilateral symmetry, whereas Me6TREN is a tris[2-(dimethylamino)ethyl]amine molecule with trivalent symmetry (see figure). To compare the activity of Me6TREN with G-CSF and plerixafor as single agents for HSC mobilization, the authors measured the content of high-proliferative–potential colony-forming units (HPP-CFU) in the blood of mice 1 hour following plerixafor administration, 12 hours after Me6TREN administration, and after 5 days of G-CSF administration and performed competitive repopulation assays by transplanting mobilized blood from mice in different treatment groups into irradiated recipients in combination with a fixed number of congenic bone marrow cells. In these experiments, mice treated with Me6TREN had significantly more HPP-CFU in their blood and superior competitive repopulating activity when transplanted into irradiated mice compared with blood mobilized with plerixafor or G-CSF. Me6TREN-mobilized progenitors contributed to long-term durable multilineage hematopoietic reconstitution in recipient mice after secondary transplantation of marrow from mice engrafted with mobilized cell, thus fulfilling the criteria for mobilization of a self-renewing HSC.
Some questions regarding this interesting new compound remain. Although Me6TREN antagonized SDF-1–induced migration of murine and human hematopoietic progenitors, antagonistic binding of Me6TREN to CXCR4 has not been established. The schedule of plerixafor administration used by Zhang et al was based upon work from Broxmeyer et al, who showed that single-agent plerixafor resulted in a 5- to 10-fold mobilization of CFU-GEM into blood 1 hour after drug administration.2 In contrast to the schedule of plerixafor used in these murine experiments, our clinical experience with plerixafor indicates that optimal mobilization of HSCs in humans usually occurs >8 hours and up to 17 hours following a subcutaneous plerixafor injection.6 Although plerixafor appears to be a pure CXCR4 antagonist and thus acts directly on CXCR4/CXCL12 binding, the authors show that Me6TREN activates the phospho-Akt, mitogen-activated protein kinase, and phospho-extracellular signal-regulated kinase pathways and induces MMP9 expression. Using radiation chimeras engrafted with MMP9-knockout bone marrow, they show that much of the mobilization induced by Me6TREN is dependent upon expression of MMP9 by hematopoietic cells. Local production of metalloproteases is implicated in disruption of adhesion molecules that tether HSCs to stromal cells, including VLA4 (see figure). It would be of interest to test whether Me6TREN acts indirectly on the CXCR4/CXCL12 axis by modulating stromal-cell expression of messenger RNA for CXCL12, the ligand for CXCR4. Thus, differences in mechanism of action of plerixafor and Me6TREN could be responsible for the observed differences in the pharmacodynamics of stem-cell mobilization following drug administration. Although Me6TREN appears to be quite potent in mobilizing HSCs in these murine models, its absolute superiority over plerixafor as a single agent for stem-cell mobilization has not been confirmed, and the optimal schedule of this new agent in humans remains to be defined.
Beyond the mobilization of hematopoietic progenitors for autologous transplantation as studied in this report, promising data published by Devine et al suggest that single-agent plerixafor can mobilize an allogeneic blood stem cell graft that may have favorable properties for donor-derived hematopoietic and immune reconstitution compared with G-CSF–mobilized products.7 It is unknown whether the use of Me6TREN in a setting of mobilization allogeneic donors would result in a graft with similar favorable immunological properties, but the discovery and preclinical development of this drug make that application an exciting new avenue for research in the field of transplantation. Finally, the availability of a new small-molecule drug with potent single-agent ability to mobilize HSCs opens the door to new clinical applications of bone marrow progenitors in nontransplant settings. We have previously shown that intermittent dosing of granulocyte-macrophage colony-stimulating factor (GM-CSF) mobilizes CD34+ cells into blood and improves endothelial dysfunction and exercise capacity in patients with severe peripheral arterial disease.8 Seiler et al demonstrated with a randomized, double-blind, placebo-controlled test that intracoronary and subcutaneous injection of GM-CSF could improve collateral flow patients with extensive coronary artery disease not eligible for coronary artery bypass surgery.9 In addition, a higher content of CD34+ cells in the blood of patients who are at high risk for myocardial infarction and death is associated with improved event-free survival.10 Thus, new drugs that mobilize progenitor cells from the blood to the bone marrow as single agents could be used on a chronic or intermittent basis in patients with vascular diseases. With progress in understanding the biology of stem-cell mobilization and with newer drugs that appear effective as single agents in mobilizing progenitor cells, research in stem cells and hematopoiesis begins to have wide clinical applications in medicine beyond stem-cell transplantation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.