In this issue of Blood, Toda et al present a shift in the paradigm of erythroid enucleation and provide novel tools to further study and optimize terminal erythroid maturation in vitro.1
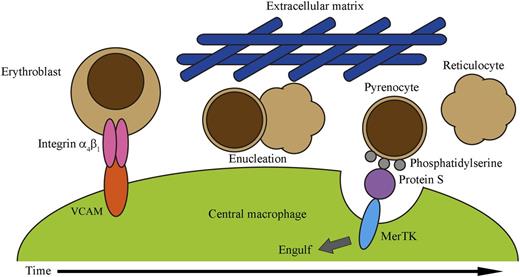
Model of interactions in erythroblastic islands. Erythroblasts bind macrophages via integrin α4β1 and VCAM interactions until they reach the stage of enucleation, at which time the erythroblast separates into a released enucleate reticulocyte and nucleated pyrenocyte. The pyrenocyte then exposes phosphatidylserine on its surface, which binds to Protein S and stimulates the MerTK receptor on the macrophage, causing its phagocytosis. See Figure 5 in the article by Toda et al that begins on page 3963.
Model of interactions in erythroblastic islands. Erythroblasts bind macrophages via integrin α4β1 and VCAM interactions until they reach the stage of enucleation, at which time the erythroblast separates into a released enucleate reticulocyte and nucleated pyrenocyte. The pyrenocyte then exposes phosphatidylserine on its surface, which binds to Protein S and stimulates the MerTK receptor on the macrophage, causing its phagocytosis. See Figure 5 in the article by Toda et al that begins on page 3963.
We have known for more than 50 years that red blood cells (RBCs) are generated in bone marrow erythroblastic islands, where erythroid precursors in various stages of maturation are bound to a central macrophage.2 Erythroblastic islands have been proposed to facilitate feedback from more mature erythroid cells to limit the expansion of younger cells, to allow macrophage signaling and nutritional support, and to eliminate the nuclear waste resulting from the synthesis of enucleated reticulocytes.3 The most striking visual aspect of this erythropoietic maturation is the last step, when the orthochromatic erythroblast goes through remarkable undulations, like a pot coming to boil, with the bulk of its cytoplasm pulling away from the nucleus until it divides into 2 very different cells.1,4 The enucleated reticulocyte continues to mature into the functional RBC. The other cell, a pyrenocyte containing the condensed nucleus surrounded by a thin layer of cytoplasm, signals the macrophage that it is the half to be eaten by exposing phosphatidylserine on its cellular membrane.5 Although erythropoiesis can be recapitulated in vitro, erythroblasts often stumble over this last step with inefficient enucleation, an issue that has challenged the ex vivo generation of RBCs.6
Adhesion between erythroid cells and macrophages involves integrin α4β1 and vascular cell adhesion molecule (VCAM), respectively, and interruption of VCAM interactions disrupts erythroblast binding to macrophages.7 During enucleation, integrin α4β1 is asymmetrically apportioned to the pyrenocyte.8 Therefore, in a simple model, the pyrenocyte retains integrin α4β1, causing it to remain behind to be eaten, whereas the reticulocyte loses integrins and can leave the island. However, Toda et al show that the simplest model is not always correct. They confirm that erythroblasts bind VCAM and that reticulocytes do not, but surprisingly, pyrenocytes also do not bind VCAM because their integrin α4β1 is in an inactive form. Instead, borrowing steps from apoptosis, Toda et al find that MerTK receptors on island macrophages bind phosphatidylserines on pyrenocyte membranes using Protein S as a linker. MerTK is a member of the TAM family of receptors that are involved in recognition and engulfment of apoptotic cells and that use specific proteins, such as protein S for MerTK, as a linker between phosphatidylserine and themselves.9 Furthermore, the authors make the important observation that increasing the viscosity of the media during ex vivo island maturation by the addition of 1% methylcellulose increased the rates of pyrenocyte consumption by macrophages. They hypothesize there is decreased diffusion of pyrenocytes during the switch from VCAM- to MerTK-based binding that may more closely emulate what occurs in the closely packed bone marrow microenvironment. Thus, a new model of terminal erythropoiesis emerges (see figure) in which the erythroblast lets go of its partner macrophage as it divides into a released reticulocyte and pyrenocyte, but the latter is caught by the macrophage and phagocytosized using mechanisms shared with apoptosis.
Along with facilitating the capture of pyrenocytes by macrophages, Toda et al observed increased enucleation on the islands when methylcellulose was added to the cultures. Under these conditions, enucleating cells were identified by their asymmetric rippling in time-lapse videos, which were followed by release of the reticulocyte. However, in typical liquid media, these rippling cells leave the island without completing enucleation. It is known that in vitro erythroid enucleation is facilitated by shear stress such as pipetting, and the authors suggest that higher viscosity media may increase shear stress generated by dynamic ruffling of the macrophages. Alternatively, increased viscosity may enhance the effectiveness of the highly active movements of the presumptive reticulocyte as it attempts to separate from the nucleus. Increased macrophage attachment may also provide a counterforce to push against. It was not reported if increased viscosity assisted erythroblast enucleation even without macrophage involvement. Whether it is macrophage fanning, reticulate struggling, or a tug of war over the pyrenocyte, this improvement of ex vivo erythroid maturation reported by Toda et al offers us new models and techniques to study erythroid enucleation.
As with any good paradigm shift, this work raises many questions for future research. One important consideration is that the authors report normal bone marrow and spleen erythropoiesis in MerTK-disrupted mice, implicating additional players. The authors identify another TAM family member, Axl, and its Gas6 linker as possible candidates to bind pyrenocytes. All of this work was performed with splenic erythroblastic islands under anemic conditions, and it remains to be determined if this process is differently regulated during steady-state conditions. Indeed, it would be interesting to know if the MerTK-disrupted mice have normal responses to erythropoietic stress. Additionally, other mediators of erythroid macrophage binding such as intercellular adhesion molecule and erythroid macrophage protein may continue to anchor the pyrenocytes during terminal maturation.3 Perhaps the most remarkable aspect of in vivo erythroblastic islands is their productiveness, producing more than 2 million new reticulocytes every second in adult humans.10 Although we can replicate erythroid maturation with erythroid cells alone, a better understanding of macrophage–erythroblast interactions may help us recapitulate the island’s efficiency making the ex vivo production of units of blood that contain trillions of RBCs a practical reality.
Conflict-of-interest disclosure: The author declares no competing financial interests.