Key Points
BH3-domain selectivity does not limit antiapoptotic capacity of prosurvival Bcl-2 proteins; the interaction profile is broad.
Intrinsically, all Bcl-2 proteins have equal antiapoptotic capacity, but proteasomal turnover limits activity of Bcl-B, Bfl-1, and Mcl-1.
Abstract
All 6 human prosurvival Bcl-2 proteins can drive cancer development and contribute to therapy resistance. However, their relative abilities to protect cells against cancer therapy were not examined previously. We report that Bcl-2, Bcl-xL, or Bcl-w consistently protected leukemic cells better than Bcl-B, Bfl-1, or Mcl-1 against a wide variety of anticancer regimens. Current thinking would attribute this to differences in their ability to bind to BH3-only proteins, Bax, and Bak. To address this, we established the first complete, quantitative cellular interaction profile of all human prosurvival Bcl-2 proteins with all their proapoptotic relatives. Binding was unexpectedly promiscuous, except for Bad and Noxa, and did not explain the differential antiapoptotic capacity of the Bcl-2 proteins. Rather, Bcl-B, Bfl-1, or Mcl-1 proved less potent due to steady-state or drug-induced proteasomal degradation. All 6 Bcl-2 proteins similarly protected against the diverse anticancer regimens when expressed at equal protein levels, in agreement with their broad interaction profile. Therefore, clinical diagnostics should include all family members and should be performed at the protein rather than at the messenger RNA level. In drug development, targeting the ubiquitination machinery of prosurvival Bcl-2 proteins will complement and potentially improve on targeting Bcl-2 protein interactions with BH3 mimetics.
Introduction
The 6 prosurvival Bcl-2 proteins Bcl-2, Bcl-B, Bcl-w, Bcl-xL, Bfl-1, and Mcl-1 inhibit the intrinsic apoptosis pathway and thereby contribute to cancer development and therapy resistance.1,2 Bcl-2 and Mcl-1 drive follicular lymphoma, chronic lymphocytic leukemia, and acute myeloid leukemia,3 whereas Bcl-B and Bfl-1 are overexpressed in various hematopoietic and solid tumors.4,5 Tumor cells are thought to be “primed for death,” due to their stressful growth conditions that mobilize many proapoptotic signals.6 The prosurvival Bcl-2 proteins counteract these signals and thereby present the Achilles’ heel of the cancer cells, which makes them important drug targets.
To find drug leads, it is important to know how the Bcl-2 protein family operates.6 Upon cellular stress, proapoptotic BH3-only proteins translocate to the mitochondria, where they activate the effector proteins Bax and Bak that subsequently homomultimerize and cause mitochondrial outer membrane permeabilization. This allows for the release of cytochrome c and other mediators that trigger caspase activation and apoptotic execution.7 The prosurvival Bcl-2 proteins inhibit apoptosis by binding to BH3-only proteins, Bax, and Bak. Their Bcl-2 homology (BH)1 to BH3 domains form a hydrophobic pocket that sequesters the BH3 domain of these proapoptotic proteins.8 Prosurvival Bcl-2 proteins exert mode 1 inhibition on BH3-only proteins and mode 2 inhibition on Bax/Bak.9 Overexpression of prosurvival Bcl-2 proteins will thus inhibit mitochondrial outer membrane permeabilization, whereas disruption of complexes between prosurvival and proapoptotic Bcl-2 proteins will promote it.
The antiapoptotic capacity of the prosurvival Bcl-2 proteins is thought to depend mainly on their ability to sequester proapoptotic Bcl-2 proteins.6,10-12 There is selectivity among these interactions. For instance, Noxa reportedly binds exclusively to Mcl-1 and Bfl-1, whereas Bad reportedly only binds to Bcl-2, Bcl-xL, and Bcl-w.6,13 Apart from a restricted binding profile, proteasomal degradation potentially also limits the antiapoptotic capacity of the prosurvival Bcl-2 proteins, as demonstrated for Mcl-1.14,15 Despite the important function of prosurvival Bcl-2 proteins, it has not been established whether binding selectivity or protein stability is the key determinant of their antiapoptotic capacity. Moreover, the capacity of the 6 different prosurvival Bcl-2 proteins to protect against different stimuli has never been compared side by side in the same model system, so their relative potency is unknown. BH3-mimetic drugs like ABT-73716 mimic the function of BH3-only proteins by binding to and inhibiting the prosurvival Bcl-2 proteins. Other strategies aim to promote degradation of prosurvival Bcl-2 proteins, in particular in the case of Mcl-1.3 To design the most effective targeting strategy, it is important to define the key determinant of the antiapoptotic capacity of the prosurvival Bcl-2 proteins, because this will identify their vulnerability.
In this study, we have accomplished this. Side-by-side analysis revealed that Bcl-2, Bcl-xL, and Bcl-w were consistently more potent than Bcl-B, Bfl-1, and Mcl-1 in protecting leukemic cells from a great variety of anticancer regimens. To try and explain this, we established the interaction profile of all 6 human prosurvival Bcl-2 proteins with all BH3-only proteins, Bax, and Bak using a novel in situ fixation technique. Interactions were less selective than anticipated and did not explain why Bcl-2, Bcl-xL, and Bcl-w consistently had a greater antiapoptotic capacity than Bcl-B, Bfl-1, and Mcl-1. It appeared that this dichotomy is not caused by BH3-domain selectivity, but by differential protein levels of the prosurvival Bcl-2 proteins at steady state or in response to anticancer regimens. When expressed at equal protein levels, all 6 Bcl-2 proteins had comparable antiapoptotic capacity to the diverse anticancer regimens, except to ABT-737 and bortezomib. This was in complete agreement with the interaction profile that defined only Bad and Noxa as selective binders. We conclude that all prosurvival Bcl-2 proteins have an equal capacity to confer resistance to diverse anticancer regimens when their expression is not limited by proteasomal turnover.
Materials and methods
Supplemental “Materials and methods,” supplemental Figures 1-10, and supplemental Table 1 are available online at the Blood Web site.
Results
Bcl-2, Bcl-w, and Bcl-xL protect better than Bcl-B, Bfl-1, and Mcl-1 against a broad range of anticancer regimens
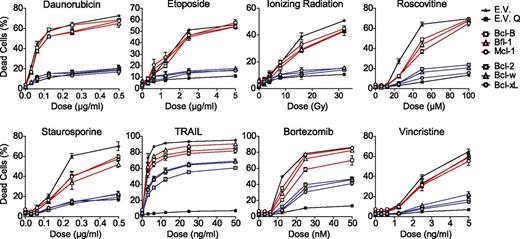
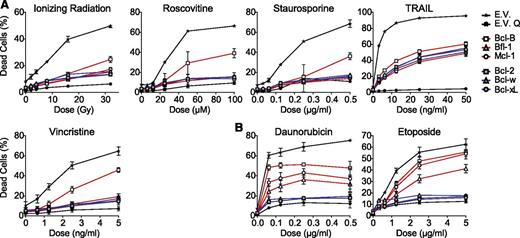
The antiapoptotic capacity of all human prosurvival Bcl-2 proteins was compared side by side in Jurkat T-acute lymphoblastic leukemia (T-ALL)–derived J16 cells.17,18 These cells were retrovirally transduced to stably express the prosurvival Bcl-2 proteins at comparable messenger RNA (mRNA) levels, whereas protein levels may differ, depending on their stability. Retroviral transduction did not result in strong overexpression, because exogenous Bcl-2 protein levels were in a similar range as endogenous Bcl-2 protein levels in 6 primary non-Hodgkin B-cell lymphomas and a B-lymphoma cell line (supplemental Figure 1). To test the antiapoptotic capacity of the Bcl-2 proteins under these conditions, the J16 cell-lines were treated with diverse anticancer regimens, ie, ionizing radiation (IR), the topoisomerase inhibitors daunorubicin and etoposide, the microtubule toxin vincristine, the cyclin-dependent kinase inhibitor roscovitine, the proteasome inhibitor bortezomib, and the death ligand TRAIL. The kinase inhibitor staurosporine was used as proapoptotic control. Apoptotic cell death was assayed after 48 hours by propidium iodide (PI) uptake and validated using the pan-caspase inhibitor Q-VD (Figure 1). Bcl-2, Bcl-w, and Bcl-xL potently protected against all stimuli except against TRAIL, which can bypass the mitochondrial apoptosis pathway18 (Figure 1). However, Bcl-B, Bfl-1, and Mcl-1 provided little or no protection against any of the stimuli (Figure 1). We considered that this dichotomy in the antiapoptotic capacity of the prosurvival Bcl-2 protein could be due to differences in their binding selectivity and/or protein stability. The contribution of both parameters has been determined in this study.
Comparative analysis of the resistance to anticancer regimens conferred by each of the 6 prosurvival Bcl-2 proteins in T-leukemic cells. J16 (Jurkat) T-ALL cells were retrovirally transduced to stably express untagged Bcl-2, Bcl-B, Bcl-w, Bcl-xL, Bfl-1, Mcl-1, or empty control vector (E.V.). All cell lines were treated with a dose range of the indicated conventional or experimental anticancer drugs, IR, or staurosporine. Cell death was assessed by PI uptake 48 hours after addition of the stimulus. E.V.-transduced cells were treated in absence or presence of the pan-caspase inhibitor Q-VD-OPH (20 µM) to demonstrate that cell death was apoptotic (E.V. Q). Data shown are mean values ± standard deviation (SD) derived from 3 independent experiments.
Comparative analysis of the resistance to anticancer regimens conferred by each of the 6 prosurvival Bcl-2 proteins in T-leukemic cells. J16 (Jurkat) T-ALL cells were retrovirally transduced to stably express untagged Bcl-2, Bcl-B, Bcl-w, Bcl-xL, Bfl-1, Mcl-1, or empty control vector (E.V.). All cell lines were treated with a dose range of the indicated conventional or experimental anticancer drugs, IR, or staurosporine. Cell death was assessed by PI uptake 48 hours after addition of the stimulus. E.V.-transduced cells were treated in absence or presence of the pan-caspase inhibitor Q-VD-OPH (20 µM) to demonstrate that cell death was apoptotic (E.V. Q). Data shown are mean values ± standard deviation (SD) derived from 3 independent experiments.
Reported Bcl-2 family binding profiles are incomplete and often inconsistent
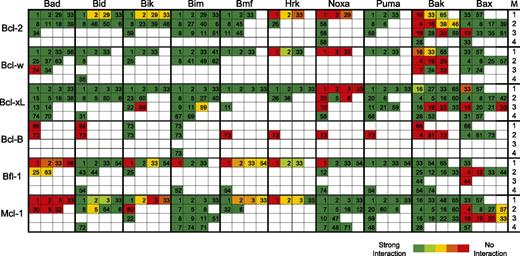
To examine the contribution of binding selectivity, we reviewed all published interactions of Bcl-2 proteins (supplemental Table 1). This included human and mouse Bcl-2 proteins, because they are used interchangeably in the field, even though conservation is not complete (supplemental Figure 2). All information is schematically represented in Figure 2. Bcl-2 protein interactions are coded with colors to represent the relative affinity and listed in 4 categories based on assay techniques.
Schematic representation of interactions between Bcl-2 family members as reported in current literature. The literature on interactions between all human and mouse Bcl-2 family members (supplemental Table 1) was reviewed as outlined in supplemental “Materials and methods.” Reported interactions were categorized according to the methodology (M) used to assess the interaction, as indicated by the numbers in the right-hand column. Categories were (1) affinity measurements in vitro, using BH3-domain peptides and isolated, C-terminally truncated prosurvival Bcl-2 proteins; (2) immunoprecipitations from cells expressing exogenous Bcl-2 family proteins; (3) immunoprecipitations from cells expressing endogenous Bcl-2 family proteins; and (4) nuclear magnetic resonance and crystal structures with recombinant proteins or biophysical measurements of interaction between full-length proteins. Boxes are color-coded for the Bcl-2 family protein pairs in each category according to the reported strength of their interaction. The number in each box refers to the pertinent publication, as cited in supplemental Table 1.
Schematic representation of interactions between Bcl-2 family members as reported in current literature. The literature on interactions between all human and mouse Bcl-2 family members (supplemental Table 1) was reviewed as outlined in supplemental “Materials and methods.” Reported interactions were categorized according to the methodology (M) used to assess the interaction, as indicated by the numbers in the right-hand column. Categories were (1) affinity measurements in vitro, using BH3-domain peptides and isolated, C-terminally truncated prosurvival Bcl-2 proteins; (2) immunoprecipitations from cells expressing exogenous Bcl-2 family proteins; (3) immunoprecipitations from cells expressing endogenous Bcl-2 family proteins; and (4) nuclear magnetic resonance and crystal structures with recombinant proteins or biophysical measurements of interaction between full-length proteins. Boxes are color-coded for the Bcl-2 family protein pairs in each category according to the reported strength of their interaction. The number in each box refers to the pertinent publication, as cited in supplemental Table 1.
The literature review did not provide sufficient information to explain our data and showed various inconsistencies. BH3-binding profiles have only been compared side by side and quantitatively by in vitro assays, employing C-terminally truncated recombinant prosurvival Bcl-2 proteins and BH3-domain peptides.6,13,19 These results do not fully agree (Figure 2 line 1) and do not necessarily reflect binding in cells, particularly because the mitochondrial membrane context is lacking.20,21 Also, removal of the membrane anchor from the prosurvival Bcl-2 proteins can alter binding properties,22-24 and BH3-peptides may not represent the full-length proteins.25,26 Most likely for these reasons, BH3-binding profiles of Bak and Bax determined in vitro differ greatly from those determined by coimmunoprecipitation from cells27 (Figure 2). However, immunoprecipitation studies are not fully consistent either (Figure 2 lines 2-3), because the detergents required to solubilize the mitochondrial membrane can induce or disrupt Bcl-2 family interactions28,29 or alter Bcl-2 protein conformation, as shown for Bcl-xL,30 Bcl-w,31 and Bax.29
Our survey revealed robust evidence for several interactions, such as for Bcl-2 with Bad and Bim, Bcl-xL with Bad and Bid, and Mcl-1 with Bim, Noxa, and Puma (Figure 2). However, findings on other interactions are less consistent, such as for Bcl-2 and Bcl-xL with Noxa and Mcl-1 with Bid, Bik, and Bmf (Figure 2). Some interactions, particularly for Hrk and Bmf, have never been tested in cells. Moreover, there is little interaction data for Bfl-1 and especially for Bcl-B (Figure 2). Thus, the Bcl-2 family interaction profile is partially inconsistent and incomplete.
Quantitative and comparative profiling reveals a broad interaction profile of most full-length Bcl-2 proteins
Realizing the limitations of available interaction data, we set out to establish the complete and quantitative binding profile of all full-length human Bcl-2 proteins in intact cells. For this purpose, N-terminally tagged prosurvival and proapoptotic Bcl-2 proteins were coexpressed pairwise in human embryonic kidney 293T cells. They were isolated by coimmunoprecipitation with antitag monoclonal antibodies (mAbs) to exclude variations in antibody affinities and were detected quantitatively with fluorochrome-conjugated antibodies and Odyssey imaging (supplemental Figure 3A). Detergent solubilization was indeed confounding, as shown for the interaction of tBid-C with all prosurvival Bcl-2 proteins. In 1% CHAPS, tBid-C was readily recovered in complex with Bcl-xL, Bfl-1, Mcl-1, and Bcl-w, whereas in 0.1% NP-40 or 0.1% Triton-X100, interactions with Bfl-1 and Mcl-1 were largely lost (supplemental Figure 3B). To avoid disruption of Bcl-2 protein interaction by detergents, cells were treated with paraformaldehyde (PFA) prior to lysis, which can link adjacent proteins in situ via a reversible covalent bond. This procedure is used in chromatin immunoprecipitation and mass spectrometry–based proteomics.32 PFA fixation indeed preserved the interactions between tBid-C and prosurvival Bcl-2 proteins that were detergent sensitive (supplemental Figure 3B). Upon mutation of critical residues in the BH3 domains of Puma, Bim, and tBid-C,9 binding to the prosurvival Bcl-2 proteins was lost, indicating that binding specificity was retained (supplemental Figure 3C). Furthermore, ABT-737 efficiently displaced Bim from Bcl-2 and Bcl-xL (supplemental Figure 4), in line with its specificity.16,17 We also tested our fixation method on endogenous Bcl-2 protein interactions. The interaction between Bim and Bcl-2 in primary B lymphoma cells was detected with similar efficiency in presence or absence of fixation and regardless of the detergent used (supplemental Figure 5A). In the same cells, we did not detect interaction between Bax and Bcl-2, either before or after fixation, when cells were lysed in CHAPS. This indicates that endogenous Bax was not activated and was in a state where its hydrophobic C terminus shields the interface required for Bcl-2 interaction8,33 Normally, Bax is activated to undergo a conformational change in response to apoptotic stimuli. This is also artificially induced postlysis by NP-40 and Triton-X100, but not by CHAPS.28 Indeed, interaction between endogenous Bax and Bcl-2 was induced by NP-40 and Triton-X100, both in nonfixed and fixed cells (supplemental Figure 5B). This further confirms the specificity of the method, because PFA did not crosslink the free pool of Bax.
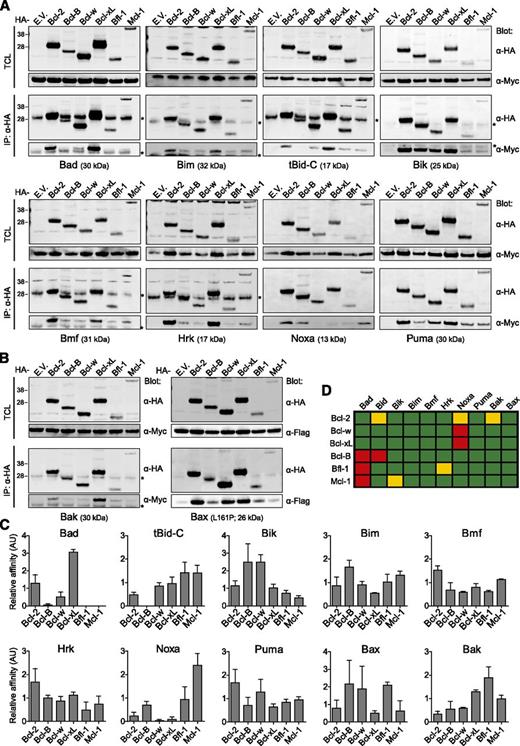
Using this novel method, we determined the quantitative cellular interaction profile of all full-length human prosurvival Bcl-2 proteins with all canonical human BH3-only proteins (Figure 3A) and Bax and Bak (Figure 3B). To assess binding to Bax, we used a constitutively active Bax mutant,33 because transfected wild-type Bax is not activated and did not bind to the prosurvival Bcl-2 proteins. The ratio of Myc- or Flag-tagged proapoptotic Bcl-2 protein was determined as recovered per hemagglutinin (HA)-tagged prosurvival Bcl-2 protein and detected on the same blot (supplemental Figure 3A). These ratios could not be used directly for quantitative comparisons, because different proteins do not transfer with equal efficiency from gel to blot34 and are not retained equally on the blot during antibody incubation.35 These differences are considerable among Bcl-2 proteins (supplemental Figure 3D) and were incorporated into the calculations of relative affinity by normalizing the binding ratios. The data obtained are depicted in graphs (Figure 3C) and schematically (Figure 3D).
In vivo interaction profile of all 6 prosurvival Bcl-2 proteins with all BH3-only proteins, Bax, and Bak. (A-B) Human embryonic kidney 293T cells were cotransfected to express each HA-tagged human prosurvival Bcl-2 protein or empty vector (E.V.) control in combination with each of the indicated Myc-tagged human BH3-only proteins (A), Myc-tagged human Bak, or Flag-tagged constitutively active (L161P) human Bax (B) in the presence of Q-VD-OPH (10 µM). After 24 hours, cells were fixed with PFA to maintain protein-protein interactions, lysed with buffer containing 1% CHAPS, and subjected to immunoprecipitation with anti-HA mAb. Total cell lysates (TCL) and immunoprecipitates (IP) were analyzed by western blotting with anti-(α)HA, α-Myc, or α-Flag mAbs. Blots are representative of 3 independent experiments. Asterisks denote the light chain of the mAb used for immunoprecipitation. (C) Signals from western blots as presented in panel A-B were quantified and the relative affinity of the proapoptotic Bcl-2 proteins for each of the prosurvival Bcl-2 proteins was expressed in arbitrary units (AU), in a normalized manner, as described in supplemental “Materials and methods.” Data are derived from 3 independent experiments. (D) Schematic representation of binding affinities calculated in panel C. Red, <0.1; yellow, <0.5; green, >0.5.
In vivo interaction profile of all 6 prosurvival Bcl-2 proteins with all BH3-only proteins, Bax, and Bak. (A-B) Human embryonic kidney 293T cells were cotransfected to express each HA-tagged human prosurvival Bcl-2 protein or empty vector (E.V.) control in combination with each of the indicated Myc-tagged human BH3-only proteins (A), Myc-tagged human Bak, or Flag-tagged constitutively active (L161P) human Bax (B) in the presence of Q-VD-OPH (10 µM). After 24 hours, cells were fixed with PFA to maintain protein-protein interactions, lysed with buffer containing 1% CHAPS, and subjected to immunoprecipitation with anti-HA mAb. Total cell lysates (TCL) and immunoprecipitates (IP) were analyzed by western blotting with anti-(α)HA, α-Myc, or α-Flag mAbs. Blots are representative of 3 independent experiments. Asterisks denote the light chain of the mAb used for immunoprecipitation. (C) Signals from western blots as presented in panel A-B were quantified and the relative affinity of the proapoptotic Bcl-2 proteins for each of the prosurvival Bcl-2 proteins was expressed in arbitrary units (AU), in a normalized manner, as described in supplemental “Materials and methods.” Data are derived from 3 independent experiments. (D) Schematic representation of binding affinities calculated in panel C. Red, <0.1; yellow, <0.5; green, >0.5.
Most interactions proved to be unselective (Figure 3C-D). Puma, Bim, and Bmf bind potently to all prosurvival Bcl-2 proteins. Other proapoptotic proteins like Bik, Hrk, Bak, and Bax also bind to many prosurvival Bcl-2 proteins but display low affinity for some. Only tBid-C, Noxa, and Bad show a complete lack of binding to certain prosurvival Bcl-2 proteins. tBid-C did not bind to Bcl-B and Bad did not bind to Bcl-B, Bfl-1, and Mcl-1, whereas Noxa did not bind to Bcl-w and Bcl-xL and bound weakly to Bcl-2 (Figure 3C-D). We conclude that Bad, Noxa, and tBid-C are the only proapoptotic Bcl-2 proteins that engage in exclusive interactions with prosurvival Bcl-2 proteins.
The dichotomy in antiapoptotic capacity of the prosurvival Bcl-2 proteins cannot be explained by selective interaction with proapoptotic family members
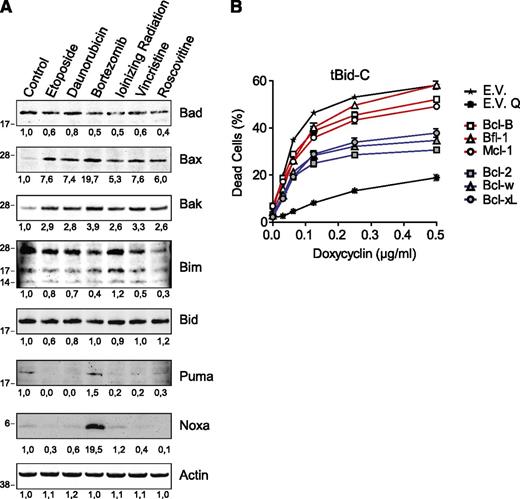
Induction of Noxa by the anticancer regimens might explain the observed dichotomy, because Noxa binds to Bcl-B, Bfl-1, and Mcl-1, not to Bcl-w and Bcl-xL, and only weakly to Bcl-2 (Figure 3D). However, among the stimuli used, only bortezomib induced Noxa expression (Figure 4A). All stimuli potently induced Bax and Bak (Figure 4A), but their binding selectivity did not match the differences in antiapoptotic potency of the prosurvival Bcl-2 proteins (Figure 3D). Protein expression of Bad, Bim, Bid, or Puma was not changed in response to any of the stimuli (Figure 4A).
Examining induction of BH3-only proteins, Bak, and Bax to explain the dichotomy in antiapoptotic capacity of the prosurvival Bcl-2 proteins. (A) J16 cells were treated with etoposide (5 µg/mL), daunorubicin (0.25 µg/mL), bortezomib (50 nM), IR (32 Gy), vincristine (5 ng/mL), or roscovitine (100 µM) for 48 hours in the presence of Q-VD-OPH (20 µM) to block apoptosis in order to maintain cell yield. Equal protein amounts of total cell lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and subjected to immunoblotting to assess expression of indicated endogenous proapoptotic proteins. Actin detection was used as a measure for equal loading. Numbers below blots indicate signal intensity normalized to the untreated control. Data are representative of 2 independent experiments. (B) J16 T-ALL cells with Dox-inducible expression of tBid-C were transduced to stably express the indicated prosurvival Bcl-2 family members or empty control vector (E.V.). Resulting cell lines were selected on antibiotics, and cell death was assessed by PI uptake 48 hours after the addition of Dox. Q-VD-OPH was taken along as a control for apoptotic cell death (E.V. Q). Data shown are mean values ± SD derived from 3 independent experiments.
Examining induction of BH3-only proteins, Bak, and Bax to explain the dichotomy in antiapoptotic capacity of the prosurvival Bcl-2 proteins. (A) J16 cells were treated with etoposide (5 µg/mL), daunorubicin (0.25 µg/mL), bortezomib (50 nM), IR (32 Gy), vincristine (5 ng/mL), or roscovitine (100 µM) for 48 hours in the presence of Q-VD-OPH (20 µM) to block apoptosis in order to maintain cell yield. Equal protein amounts of total cell lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and subjected to immunoblotting to assess expression of indicated endogenous proapoptotic proteins. Actin detection was used as a measure for equal loading. Numbers below blots indicate signal intensity normalized to the untreated control. Data are representative of 2 independent experiments. (B) J16 T-ALL cells with Dox-inducible expression of tBid-C were transduced to stably express the indicated prosurvival Bcl-2 family members or empty control vector (E.V.). Resulting cell lines were selected on antibiotics, and cell death was assessed by PI uptake 48 hours after the addition of Dox. Q-VD-OPH was taken along as a control for apoptotic cell death (E.V. Q). Data shown are mean values ± SD derived from 3 independent experiments.
To further address the importance of BH3-domain binding selectivity for antiapoptotic capacity, we tested activity of the prosurvival Bcl-2 proteins against the promiscuous binder tBid-C (Figure 3D). J16 cells endowed with doxycycline (Dox)-inducible tBid-C17 were transduced to express each of the 6 prosurvival Bcl-2 family members or empty vector and tested for apoptosis sensitivity. Again, Bcl-2, Bcl-w, and Bcl-xL inhibited tBid-C–induced apoptosis more potently than Bcl-B, Bfl-1, or Mcl-1 (Figure 4B). The collective data indicate that the differential antiapoptotic capacity of Bcl-2, Bcl-w, and Bcl-xL versus Bcl-B, Bfl-1, and Mcl-1 cannot be explained by differences in binding to BH3-only proteins, Bax, or Bak.
The antiapoptotic capacity of the prosurvival Bcl-2 proteins correlates with their half-life
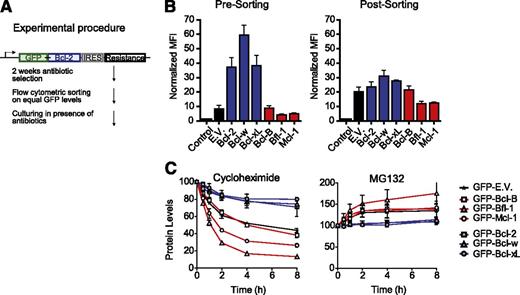
We next addressed the role of Bcl-2 protein turnover in antiapoptotic capacity. It is known that Mcl-1, Bfl-1, and Bcl-B are subject to ubiquitin-mediated degradation,14,22,36,37 but Bcl-238,39 and Bcl-xL40 can also be ubiquitinated and degraded in response to apoptotic stimuli. To test the role of Bcl-2 protein turnover, J16 cells were used that express the prosurvival Bcl-2 proteins as N-terminal GFP fusions (Figure 5A). GFP fusion permits measure and equalization of Bcl-2 protein levels on basis of GFP fluorescence intensity and does not affect antiapoptotic activity of the Bcl-2 proteins.41
Prosurvival Bcl-2 proteins differ greatly in steady-state expression due to proteasomal turnover. (A) Schematic overview of the procedure to make J16 cell lines stably expressing N-terminal GFP fusions of the prosurvival Bcl-2 proteins at equal levels. (B) J16 cell lines transduced with constructs encoding N-terminal GFP fusions of the individual prosurvival Bcl-2 proteins as depicted in (A) were selected with antibiotics for 2 weeks, flow cytometrically sorted on equal GFP fluorescence intensity, and cultured on antibiotics for another week. Mean fluorescence intensity (MFI) of GFP was determined just before sorting (left) or 1 week after sorting (right) and normalized to untransduced cells (control). Data are shown as mean values ± SD of 3 independent transductions. (C) J16 cell lines expressing GFP fusions of the indicated prosurvival Bcl-2 proteins or empty vector (E.V.) were sorted on equal GFP fluorescence intensity and treated with the protein-synthesis inhibitor cycloheximide (50 µg/mL) or proteasome inhibitor MG132 (50 µM). At the indicated time points, GFP fluorescence intensity was assessed for each cell line by flow cytometry and expressed relative to its fluorescence intensity at the 0-hour time point, which was set at 100%. GFP expressed from the empty vector (E.V.) was a target for proteasomal degradation (C). This was due to a cloning artifact, only present for the E.V. but not for the fusion proteins, that endowed GFP-E.V. with a destabilizing C-terminal amino acid stretch (results not shown). Data are from 3 independent experiments and show mean values ± SD.
Prosurvival Bcl-2 proteins differ greatly in steady-state expression due to proteasomal turnover. (A) Schematic overview of the procedure to make J16 cell lines stably expressing N-terminal GFP fusions of the prosurvival Bcl-2 proteins at equal levels. (B) J16 cell lines transduced with constructs encoding N-terminal GFP fusions of the individual prosurvival Bcl-2 proteins as depicted in (A) were selected with antibiotics for 2 weeks, flow cytometrically sorted on equal GFP fluorescence intensity, and cultured on antibiotics for another week. Mean fluorescence intensity (MFI) of GFP was determined just before sorting (left) or 1 week after sorting (right) and normalized to untransduced cells (control). Data are shown as mean values ± SD of 3 independent transductions. (C) J16 cell lines expressing GFP fusions of the indicated prosurvival Bcl-2 proteins or empty vector (E.V.) were sorted on equal GFP fluorescence intensity and treated with the protein-synthesis inhibitor cycloheximide (50 µg/mL) or proteasome inhibitor MG132 (50 µM). At the indicated time points, GFP fluorescence intensity was assessed for each cell line by flow cytometry and expressed relative to its fluorescence intensity at the 0-hour time point, which was set at 100%. GFP expressed from the empty vector (E.V.) was a target for proteasomal degradation (C). This was due to a cloning artifact, only present for the E.V. but not for the fusion proteins, that endowed GFP-E.V. with a destabilizing C-terminal amino acid stretch (results not shown). Data are from 3 independent experiments and show mean values ± SD.
After antibiotic selection of the newly made cell lines (Figure 5A), Bcl-2, Bcl-w, and Bcl-xL were expressed at significantly higher levels than Bcl-B, Bfl-1, and Mcl-1 (Figure 5B). To equalize expression levels, the cell lines were sorted on equal GFP fluorescence intensity (Figure 5B; supplemental Figure 6). GFP-Bcl-B was partly proteolyzed to GFP only. Therefore, GFP fluorescence intensity denoted protein levels of GFP-Bcl-B less reliably than of the other GFP-tagged prosurvival Bcl-2 proteins (supplemental Figure 6). To assess turnover of the prosurvival Bcl-2 proteins, protein synthesis was inhibited with cycloheximide and protein levels were monitored by flow cytometry over time. GFP-Bcl-B, GFP-Bfl-1, and GFP-Mcl-1 proved to be unstable proteins, with half-lives between 1.5 and 4 hours (Figure 5C). In contrast, Bcl-2, Bcl-w, and Bcl-xL were stable, with half-lives of about 20 hours. This agrees with published data on the untagged proteins,14,22,36,37 indicating that GFP fusion did not influence the turnover of the Bcl-2 proteins. Accordingly, the turnover of GFP-Bcl-2 and GFP-Mcl-1 was virtually identical to that of endogenous Bcl-2 and Mcl-1 in the same cells (supplemental Figure 7). Bcl-B, Bfl-1, and Mcl-1 were degraded by the proteasome, because they were stabilized by the proteasome inhibitor MG132 (Figure 5C). Collectively, these data indicate that Bcl-B, Bfl-1, and Mcl-1 are highly unstable as compared with Bcl-2, Bcl-w, and Bcl-xL due to rapid proteasomal degradation, which correlates with their antiapoptotic capacity.
Steady-state or stimulus-induced degradation determines the capacity of prosurvival Bcl-2 proteins to inhibit apoptosis
To determine whether protein turnover was the decisive factor for antiapoptotic capacity, the prosurvival Bcl-2 proteins were again compared for their antiapoptotic capacity, but now at equal protein levels. The J16 cell lines sorted for equal expression of the GFP-Bcl-2 fusion proteins (Figure 5B) were exposed to the same anticancer regimens used before (Figure 1). Strikingly, all prosurvival Bcl-2 proteins now provided equal protection to IR, vincristine, roscovitine, staurosporine, and TRAIL (Figure 6A), except Bcl-B. Bcl-B blocked less effectively, because loss of GFP from the GFP-Bcl-B fusion protein (supplemental Figure 6) did not allow us to accurately equalize Bcl-B protein levels on basis of the GFP signal.
Steady-state or stimulus-induced degradation of prosurvival Bcl-2 proteins limits their proapoptotic activity. (A) J16 cell lines transduced to express the individual prosurvival Bcl-2 proteins as N-terminal GFP fusions and sorted on equal protein levels (as in Figure 5A) were treated with a dose range of the indicated proapoptotic regimens. Cell death was assessed by PI uptake after 48 hours. Empty vector (E.V.) transduced cells were used as control and treated with Q-VD-OPH (E.V. Q) to show that cell death was apoptotic. (B) As in panel A, using etoposide and daunorubicin as proapoptotic regimens. Data shown are mean values ± SD derived from 3 independent experiments.
Steady-state or stimulus-induced degradation of prosurvival Bcl-2 proteins limits their proapoptotic activity. (A) J16 cell lines transduced to express the individual prosurvival Bcl-2 proteins as N-terminal GFP fusions and sorted on equal protein levels (as in Figure 5A) were treated with a dose range of the indicated proapoptotic regimens. Cell death was assessed by PI uptake after 48 hours. Empty vector (E.V.) transduced cells were used as control and treated with Q-VD-OPH (E.V. Q) to show that cell death was apoptotic. (B) As in panel A, using etoposide and daunorubicin as proapoptotic regimens. Data shown are mean values ± SD derived from 3 independent experiments.
In the case of daunorubicin and etoposide, Bcl-2, Bcl-w, and Bcl-xL still inhibited the apoptotic response better than Bcl-B, Bfl-1, and Mcl-1 (Figure 6B). Renewed sorting of the cell lines to precisely equalize protein levels (supplemental Figure 8A-B) still proved Mcl-1 inferior to Bcl-w at the 48-hour time point (supplemental Figure 8C). At the 24-hour time point, however, all prosurvival Bcl-2 proteins inhibited the response to a similar extent (supplemental Figure 9). This suggested that Bcl-B, Bfl-1, and Mcl-1 were degraded in response to drug treatment. Indeed, protein levels of Bfl-1 and Mcl-1 decreased progressively after drug addition, whereas levels of Bcl-2, Bcl-w, and Bcl-xL remained unchanged (supplemental Figure 10). We conclude that all 6 Bcl-2 proteins can provide similar protection against diverse anticancer regimens when expressed at equal protein levels, in agreement with their broad interaction profile. Steady-state or drug-induced proteasomal degradation, but not BH3-domain selectivity, is the limiting factor in their antiapoptotic capacity.
BH3-domain selectivity can determine antiapoptotic capacity in response to selective stimuli.
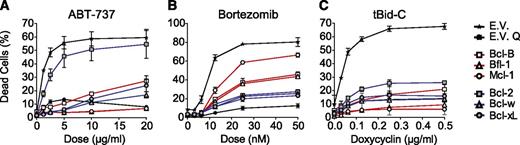
The prosurvival Bcl-2 proteins did not bind with equal affinity to Bad, Noxa, or tBid-C. Therefore, BH3-domain selectivity may still be decisive for the antiapoptotic capacity of the prosurvival Bcl-2 molecules when these selective BH3-only proteins are at play. To examine the consequences of treatment with a selective anticancer drug, the J16 cell lines that expressed the prosurvival Bcl-2 proteins at equal levels were treated with the BH3-mimetic ABT-737 (Figure 7A). As predicted, cell death was most potently blocked by the nontargeted Bfl-1 and Mcl-1. The nontargeted Bcl-B blocked to a lesser extent, for the reason mentioned above. Bcl-2 provided almost no resistance, whereas Bcl-w and Bcl-xL did, in accordance with the finding that in vivo, Bcl-2 is the preferred target of ABT-737 among these 3.17,42 We also used the proteasome inhibitor bortezomib that potently induces Noxa (Figure 4B) and has been shown to kill multiple cell types including Jurkat by means of Noxa.43,44 In line with their binding selectivity for Noxa, Bcl-2, Bcl-xL, and Bcl-w provided potent protection, whereas Bcl-B, Bfl-1, and especially Mcl-1 did not (Figure 7), even though bortezomib stabilized them (supplemental Figure 10).
BH3-domain selectivity can determine antiapoptotic capacity when selective BH3-only proteins Bad or Noxa are exclusively involved. (A-B) J16 cell lines transduced to express the individual prosurvival Bcl-2 proteins as N-terminal GFP fusions and sorted on equal protein levels (as in Figure 5A) were treated with a dose range of ABT-737 (A) or bortezomib (B). Cell death was assessed by PI uptake after 48 hours. Empty vector (E.V.) transduced cells were used as control and treated with QVD-OPH (E.V. Q) to show that cell death was apoptotic. (C) J16 cell line with Dox-inducible expression of tBid-C was transduced to express the individual prosurvival Bcl-2 proteins as N-terminal GFP fusions and the resulting cell lines were sorted on equal protein levels (as in Figure 5A). Cells were treated with the indicated dose range of Dox, and cell death was assessed by PI uptake 48 hours after Dox addition. Data shown are mean values ± SD derived from 3 independent experiments.
BH3-domain selectivity can determine antiapoptotic capacity when selective BH3-only proteins Bad or Noxa are exclusively involved. (A-B) J16 cell lines transduced to express the individual prosurvival Bcl-2 proteins as N-terminal GFP fusions and sorted on equal protein levels (as in Figure 5A) were treated with a dose range of ABT-737 (A) or bortezomib (B). Cell death was assessed by PI uptake after 48 hours. Empty vector (E.V.) transduced cells were used as control and treated with QVD-OPH (E.V. Q) to show that cell death was apoptotic. (C) J16 cell line with Dox-inducible expression of tBid-C was transduced to express the individual prosurvival Bcl-2 proteins as N-terminal GFP fusions and the resulting cell lines were sorted on equal protein levels (as in Figure 5A). Cells were treated with the indicated dose range of Dox, and cell death was assessed by PI uptake 48 hours after Dox addition. Data shown are mean values ± SD derived from 3 independent experiments.
According to the interaction profile, tBid-C does not bind to Bcl-B. tBid-C induces apoptosis by direct activation of Bax or Bak,45 and prosurvival Bcl-2 proteins can inhibit tBid-C action directly (mode 1) or by binding to activated Bax and Bak (mode 2).9 Consequently, even though Bcl-B does not bind tBid-C, it should still inhibit tBid-C–induced apoptosis by binding to Bax and Bak. To examine this, the J16 cell line with Dox-inducible tBid-C expression (Figure 4B) was transduced and sorted to express each of the 6 N-terminally GFP-tagged prosurvival Bcl-2 proteins at equal protein levels. The ability of Bfl-1 and Mcl-1 to protect against tBid-C–induced apoptosis was now equal to that of Bcl-2, Bcl-xL, and Bcl-w (Figure 7C), as opposed to the earlier experiment in which their protein levels were not controlled (Figure 4). Bcl-B also provided similar protection to tBid-C (Figure 7C), in agreement with mode 2 inhibition.9 The collective data argue that binding selectivity can determine the antiapoptotic capacity of the prosurvival Bcl-2 proteins when stimuli mimic Bad or Noxa or exclusively act via these selective BH3-only proteins,
Discussion
The mode of action of the prosurvival Bcl-2 family members is keenly researched because of their important role in tumorigenesis and treatment resistance.1,2,46 According to our study, all prosurvival Bcl-2 proteins are equally potent in providing resistance to diverse anticancer regimens when expressed at equal levels. To determine the importance of BH3-domain selectivity for the antiapoptotic capacity of the prosurvival Bcl-2 proteins, we examined their binding to all canonical human BH3-only proteins, Bax, and Bak. This was done in intact cells, in a quantitative manner, after reversible, in situ fixation. The resulting interaction profile pointed out that Bcl-2 family interactions are much less selective than previously anticipated. Activated Bax, Bak, and the majority of the BH3-only proteins can bind to all of the prosurvival Bcl-2 proteins, albeit with different affinities. Only tBid-C, Bad, and Noxa displayed explicit binding selectivity: tBid-C did not bind to Bcl-B and Bad did not bind to Bcl-B, Bfl-1, and Mcl-1, whereas Noxa did not bind to Bcl-w and Bcl-xL. Noxa and Bak did bind with low affinity to Bcl-2, which has been disputed (Figure 2; supplemental Table 1). Indeed, both interactions were recently found in vivo and contributed to apoptosis resistance.43,47 These authors reported that naturally occurring Bcl-2 variants have significantly different affinities for Noxa and Bak, possibly explaining previous conflicting findings.
The established binding profile did not explain the dichotomy in antiapoptotic capacity of the prosurvival Bcl-2 proteins. Rather, we found a correlation between the antiapoptotic capacity and proteasomal turnover of the prosurvival Bcl-2 proteins. Bcl-B, Bfl-1, and Mcl-1 were instable and protected J16 cells less well than the stable proteins Bcl-2, Bcl-xL, and Bcl-w against a wide variety of proapoptotic stimuli. By expressing all 6 prosurvival Bcl-2 proteins at comparable protein levels, we proved that the high turnover and consequent low protein level of Bcl-B, Bfl-1, and Mcl-1 was the key factor that limited their ability to confer apoptosis resistance. The notion that stability can limit the antiapoptotic capacity of prosurvival Bcl-2 proteins is accepted. However, we are the first to show that the high-turnover Bcl-2 proteins Bcl-B, Bfl-1, and Mcl-1 can protect cells in equal measure against a wide variety of anticancer regimens as Bcl-2, Bcl-xL, and Bcl-w when their protein levels are equal. Thus, protein levels, rather than intrinsic structural properties, limit the antiapoptotic capacity of Bcl-B, Bfl-1, and Mcl-1. For the high-turnover Bcl-2 proteins, changes in mRNA levels or translation efficiency can also rapidly alter protein levels. As a case in point, Wei et al48 reported that diverse anticancer drugs can limit endogenous Mcl-1 expression via such mechanisms. We have not examined such effects here, because the exogenous mRNAs of the prosurvival Bcl-2 family members do not contain the endogenous regulatory sequences. The dichotomy in antiapoptotic capacity of the prosurvival Bcl-2 proteins is not restricted to therapy resistance. An Eµ-Myc–driven leukemogenesis model revealed that Bcl-2, Bcl-xL, and Bcl-w also have a greater oncogenic potency than Bcl-B, Bfl-1, and Mcl-1,2 whereas stabilized versions of Bcl-B36 and Bfl-122 were as potent in accelerating tumorigenesis as Bcl-xL. Interestingly, a stabilized form of Mcl-1 expressed from the endogenous gene locus was recently found to largely rescue the dramatic developmental deficiencies of Bcl-2−/− mice.49 This further supports the concept that the antiapoptotic potency of Mcl-1 is similar to that of Bcl-2 if its degradation is impeded.
The broad Bcl-2 protein interaction profile fully supports the concept that intrinsically, all prosurvival Bcl-2 proteins have equal antiapoptotic capacity. This exception is when stimuli exclusively induce the selective binders Bad or Noxa or when selective BH3 mimetics are used. At equal expression levels, only Bcl-2, Bcl-xL, and Bcl-w conferred resistance to bortezomib and only Bcl-B, Bfl-1, and Mcl-1 conferred resistance to the BH3 mimetic ABT-737. Even though Bcl-B could not bind to tBid-C, it could inhibit tBid-C–induced apoptosis by binding to Bax or Bak. Anticancer regimens will generally not selectively engage Bad or Noxa but will engage multiple BH3-only proteins, including those with a broad binding profile. For instance, in primary lymphocytes, Noxa only made a small contribution to the apoptotic response to DNA-damaging agents.50 Furthermore, the p53-dependent response to etoposide in Eµ-Myc lymphomas relied on the combined action of Noxa, Puma, and Bim.51
Our findings emphasize that clinical diagnostics should include all Bcl-2 family members, because all have potentially equal roles in therapy resistance when overexpressed. Clearly, mRNA levels of Bcl-B, Bfl-1, and Mcl-1 are unlikely to reflect their role in treatment resistance, because expression is also controlled at the protein level. This might explain why it has proven difficult to predict the celluar response to ABT-737 on basis of mRNA levels of the prosurvival Bcl-2 proteins.52 It will be much more informative to use protein levels of the prosurvival Bcl-2 molecules for the prediction of treatment response, as recently demonstrated for Bcl-B.53
Our data imply that Bcl-B, Bfl-1, and Mcl-1 proteins can contribute to tumor development and therapy resistance as potently as Bcl-2, Bcl-xL, and Bcl-w when their protein levels increase due to mutations in their degradation machinery. Indeed, loss-of-function mutations in the gene encoding the Mcl-1 ubiquitin ligase FBW7 occur in primary human carcinomas and correlate with increased Mcl-1 expression.15 Moreover, this Mcl-1 protein accumulation caused resistance to antitubulin chemotherapeutics.15 Also in human T-ALL, loss of FBW7 function led to increased Mcl-1 expression and resistance to diverse anticancer regimens, including ABT-737.54 Our data highlight a level of control that can potentially be targeted. Compounds that would inactivate specific deubiquitination enzymes or activate ubiquitin ligases might kill tumor cells that depend on Bcl-B, Bfl-1, or Mcl-1 for their survival. Accordingly, an inhibitor of Mcl-1’s deubiquitinase USP9x decreased Mcl-1 protein levels and induced apoptosis in chronic myeloid leukemia.55 Our work indicates that such approaches are also valid in malignancies with overexpression of Bcl-B or Bfl-1.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs H. Walczak, H. Ovaa, H. G. Wang, and O. van Tellingen for reagents; F. van Diepen and A. Pfauth for experimental assistance; and Drs S. Tait, I. Verbrugge, J. P. Medema, and S. Kaufmann for critical reading of the manuscript and/or experimental advice.
This work was supported by grants from the Dutch Cancer Society (M.V. and J.B.) and by grant ECHO 700.57.008 from the Netherlands Organization for Scientific Research.
Authorship
Contribution: R.W.R. conceived the project with input from J.B. and M.V.; R.W.R. performed experiments, evaluated data, and wrote the paper; M.P., B.v.d.K., E.d.V., and R.B. performed experiments and evaluated data; M.V. evaluated data; and J.B. evaluated data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jannie Borst, Division of Immunology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, the Netherlands; e-mail: j.borst@nki.nl.
References
Author notes
B.v.d.K., E.d.V., and M.P. contributed equally to this study.