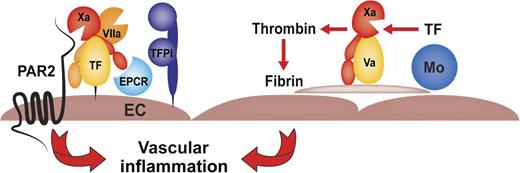
SCD vascular inflammation is caused by microthrombotic events of TF expressed by myelomonocytic cells (Mo) and amplified by abnormal red blood cells and/or activated platelets. Noncoagulant endothelial cell (EC) TF-dependent FXa-PAR2 signaling contributes independently to inflammation by increasing circulating levels of IL-6.
SCD vascular inflammation is caused by microthrombotic events of TF expressed by myelomonocytic cells (Mo) and amplified by abnormal red blood cells and/or activated platelets. Noncoagulant endothelial cell (EC) TF-dependent FXa-PAR2 signaling contributes independently to inflammation by increasing circulating levels of IL-6.
Vascular occlusion is a major complication in sickle cell disease (SCD), leading to stroke and infarcts in other organs. The abnormal flow properties of sickle red blood cells trigger complex multicellular interactions causing repeated reperfusion injury, chronic inflammation, and endothelial dysfunction. A unique feature of the inflamed endothelium in SCD is expression of the coagulation initiator tissue factor (TF), which is normally absent from the endothelial barrier but induced in pulmonary vein endothelial cells through direct hemolysis-dependent and/or indirect leukocyte inflammation-dependent mechanisms.2 Additional precipitating factors contribute to vaso-occlusive crises, including reactive oxygen species (ROS) and concomitant depletion of vascular protective nitric oxide leading to increased platelet activation. Of note, TF cellular procoagulant activity is tightly regulated but increased markedly by cell-damage signals, leading to ROS-dependent release of TF+ procoagulant microparticles3 that are detectable in the blood of SCD patients during crisis.
In a previous study, the group of Pawlinski4 demonstrated that TF is expressed at increased levels by monocytes and neutrophils and responsible for the chronic procoagulant state in mouse models of SCD. Accordingly, elevated markers of inflammation, including neutrophil infiltration of the lungs, increased lung messenger RNA levels of chemokines MCP-1 and CXCL1, and circulating levels of soluble vascular cell adhesion molecule (VCAM), were sensitive to antibody blockade of the procoagulant function of TF but independent of vessel-wall TF. However, endothelial cell–specific deletion of TF in the transplant setting of this mouse model reduced the elevated levels of one particular inflammatory cytokine, interleukin-6 (IL-6), without changing markers of intravascular coagulation. These data indicated that TF as a dual receptor with procoagulant and signaling functions5 makes distinct contributions to SCD pathology. The present study further elucidates pathogenic mechanisms in this chronic inflammatory disease with unmet therapeutic need.
Sparkenbaugh et al1 evaluated different therapeutic approaches to block TF-initiated coagulation in the established mouse model of SCD and focused on administering new oral anticoagulants that are currently finding wider clinical use. The thrombin inhibitor dabigatran was effective in reducing lung leukocyte infiltration, confirming the previously demonstrated pharmacologic benefits of blocking TF activity. Similarly, the FXa inhibitor rivaroxaban was as effective as dabigatran in reducing intravascular coagulation abnormalities and lung neutrophil infiltration, although the chosen dose of rivaroxaban interfered with experimental hemostatic clot formation to a lesser degree as observed in mice stably anticoagulated with the thrombin inhibitor. Because genetic deletion of the thrombin receptor, protease-activated receptor 1, in nonhematopoietic cells had no beneficial effects in this mouse model, these data indicate that coagulation and/or platelet activation precipitating microthrombotic events were responsible for these aspects of lung inflammation in SDC and preventable by anticoagulant therapy.
Intriguingly, in the previous study, TF antibody blockade decreased levels of soluble VCAM,4 but this endothelial cell activation marker was unaffected by anticoagulation with dabigatran or rivaroxaban. Because genetic ablation of TF from endothelial cells had no effect on soluble VCAM levels in SCD mice, TF synthesized by myeloid or other vessel wall cells appears to affect the vasculature in SCD through additional noncoagulant effects. Although not resolved here, possible mechanisms to be considered are coagulation-independent direct signaling of the TF–factor VIIa (FVIIa) complex5 leading to paracrine effects on the endothelium or recently established TF trans-signaling pathways. For example, hypoxic endothelial cells can be activated through microparticles carrying TF-FVIIa,6 and the noncoagulant and soluble alternatively spliced TF ligates endothelial integrins and induces leukocyte adhesion molecules, including VCAM.7 Mechanistic studies into these noncoagulant functions of TF may yield clues for other therapeutic avenues to further improve vascular dysfunction in SCD.
The complex cellular interactions contributing to the pathology of SCD may bring together in a locally coordinated fashion proinflammatory inducers of endothelial cell TF2 and procoagulant myelomonocytic cells (see figure). Consistent with prior findings that endothelial cell TF is responsible for increasing circulating IL-6 levels without causing coagulation activation in SCD,4 Sparkenbaugh et al1 show that the thrombin inhibitor dabigatran was ineffective to normalize this particular marker of inflammation. Surprisingly, IL-6 levels were markedly reduced by the direct FXa inhibitor rivaroxaban as well as by deletion of the FXa signaling receptor protease-activated receptor 2 (PAR2) from nonhematopoietic cells. These data indicate that autocrine signaling in the initiation phase of coagulation contributes to the pathogenesis of vascular inflammation in this mouse model of SCD. In vitro studies have shown that cytokine-stimulated endothelial cells receive signals from FXa that is newly generated by the induced TF-FVIIa complex.8 Signaling of the TF coagulation initiation complex furthermore requires the endothelial cell protein C receptor (EPCR).9 EPCR limits the generation of procoagulant FXa on endothelial cells,10 and the endothelial cell–expressed TF signaling complex is under anticoagulant control by the physiological TF pathway inhibitor (TFPI),8 possibly explaining how TF-initiated protease signaling through PAR2 may occur with minimal systemic coagulation activation.
The delineated function of TF in FXa and PAR2-dependent vascular inflammation may overlap with pathological mechanisms underlying pulmonary hypertension, a known complication of SCD.1 The dual roles of TF in SCD pathology suggest that selectively targeting FXa provides an added benefit not only by interrupting microthrombosis and its inflammatory sequela but also by reducing direct endothelial proinflammatory signaling. The identification of IL-6 as a potential biomarker for the latter should facilitate clinical studies that are required to validate the human relevance of these results from animal models of SCD. FXa-selective oral anticoagulants have demonstrated efficacy equivalent to vitamin K antagonists in the prophylaxis of venous thromboembolism while reducing the risk of fatal and cerebral hemorrhage. This favorable profile may encourage clinical trials with these inhibitors in SCD where microthrombotic events are important contributors to vaso-occlusive complications.
Conflict-of-interest disclosure: The author declares no competing financial interests.