In this issue of Blood, Trottier et al. demonstrate that the outcome of allogeneic stem cell transplantation (HCT) was not significantly impacted by iron overload, as defined by liver magnetic resonance imaging (R2-MRI). This study implies that treatment decisions for iron overload should not be based on ferritin alone and that assessment of the liver iron content (LIC) may be a more accurate measure of body iron stores.1
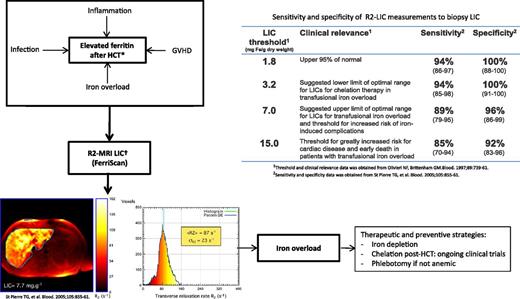
Role of R2-MRI in the quantification of LIC in HCT recipients. GVHD, graft-versus-host disease. Iron overload: transfusion, dyserythropoiesis, associated genetic factors (HEF mutations); Non-iron-overload etiology: inflammation (GVHD, infections, radiation), metabolic syndrome (immunosuppression), hepatitis (viral, drugs, GVHD, radiation), associated ETOH usage; *Elevated ferritin; †LIC predicts body iron stores, changes in LIC show changes body iron with chelation therapy, calculate iron balance, predicts risk of hepatic complications and risk of extra-hepatic complications. Liver R2 panels are reproduced from Figure 4 in St Pierre et al2 by permission.
Role of R2-MRI in the quantification of LIC in HCT recipients. GVHD, graft-versus-host disease. Iron overload: transfusion, dyserythropoiesis, associated genetic factors (HEF mutations); Non-iron-overload etiology: inflammation (GVHD, infections, radiation), metabolic syndrome (immunosuppression), hepatitis (viral, drugs, GVHD, radiation), associated ETOH usage; *Elevated ferritin; †LIC predicts body iron stores, changes in LIC show changes body iron with chelation therapy, calculate iron balance, predicts risk of hepatic complications and risk of extra-hepatic complications. Liver R2 panels are reproduced from Figure 4 in St Pierre et al2 by permission.
Although a low serum ferritin is an accurate measure of iron deficiency, there is no accurate serum or plasma marker for body-iron overload. Previously, liver biopsy with iron determination by mass spectroscopy was the method of choice, but biopsy carries risk and is not suited for population-based studies. Several methods are available for this purpose and have, over time, been dominated by noninvasive techniques. These include iron-specific MRI, which has been proven to accurately measure not only liver iron but also iron accumulation in the heart, pituitary, pancreas, and thyroid with no side effects. There are two validated MRI methods for quantitating the liver iron burden: the FerriScan and T2 methods.1,2 The noninvasive R2-MRI technique (FerriScan) is highly sensitive and specific for estimating LIC and is approved by the Food and Drug Administration for routine clinical use.
In this issue of Blood, Trottier et al1 report on an important study that compares the outcome of patients with or without iron overload as measured by R2-MRI of the liver. They found no significant difference in the 1-year probability of survival, nonrelapse mortality, graft-versus-host disease, organ failure, infections, or hepatic veno-occlusive disease in the 2 groups. Moreover, the recovery of natural killer, T cells, and CD4+, CD8+, and regulatory T cells was not delayed in patients with iron overload. These observations are in contrast to multiple previous reports that used elevated ferritin as a surrogate measure of iron overload after HCT and found an association between high serum ferritin levels and increased risk of transplant-related morbidity, mortality, and delayed immune recovery.3,4
From the diagnostic standpoint, the management of high serum ferritin levels after HCT should be based on the pathophysiological mechanisms underlying the development of hyperferritinemia. This knowledge is essential for differentiating increased serum ferritin due to iron overload from “non-iron-overload” situations (see figure).
The effects of persistent iron overload on the long-term morbidity of HCT survivors have not been fully investigated, mostly because of the lack of a method for population-based testing of the iron burden. Based on experience in children with hemoglobinopathies, persistent iron overload may be a risk factor for long-term organ damage and dysfunction.3-5 In patients with myelodysplastic syndromes or acute leukemia undergoing HCT, pretransplantation transfusion history and high serum ferritin have a significant unfavorable prognostic value, resulting in significant decrease in survival and increase in late nonrelapse mortality.6-8 Although prospective studies to evaluate the impact of iron overload on posttransplantation morbidity and mortality are needed, removal of excess iron constitutes an important goal and might decrease late effects after HCT.
The current study provides insight into the applicability of noninvasive and more reliable methods to evaluate iron stores in selected patients who may require treatment of iron overload after HCT. It also identifies patients with high ferritin but normal iron stores who would otherwise not require iron chelation.
Although HCT is being increasingly used as a curative therapy for severe disorders of the hematopoietic and immune systems, the focus has moved over time from immediate recipient survival to long-term follow-up. Thus far, only a few studies have investigated the effects of persistent iron overload on the long-term morbidity of HCT in nonthalassemic recipients. Iron overload has been linked to multiple long-term complications after HCT, including late cardiovascular, development of a second malignancy, and endocrine and neurologic dysfunction.3,4,9 The current study follows patients for 1-year posttransplant and evaluates the role of R2-MRI in the estimation of iron overload. However, the impact of iron overload on long-term survival and late complications requires further investigation. Whereas current guidelines recommend ferritin measurement at annual post-HCT visits,9,10 the use of R2-MRI in patients found to have elevated ferritin levels might provide a more accurate measure of iron overload and identify patients at greater risk of late complications. This information might help in the design of preventive strategies to reduce the risk of long-term complications after HCT.
In contrast to multiple previous retrospective studies, the current study reassures clinicians that it is acceptable to avoid chelation therapy in patients with pretransplant iron overload while more research is conducted in this area. This is especially relevant because chelation therapy is not without risks of toxicity. Phlebotomy treatment is a simple and effective approach to remove excess iron from tissues; however, a proportion of HCT recipients may not be eligible for phlebotomy because of coexisting anemia. Although deferoxamine has been available for many years and considered as the standard of care in this setting, this compound has several limitations. These include lack of oral absorption and a very short half-life when administered parenterally. Deferiprone is more effective than deferoxamine and was shown to improve asymptomatic myocardial siderosis in patients with β-thalassemia major. However, its use has not been investigated in HCT recipients. Deferasirox is an oral iron chelator that has been used in post-HCT patients; however, given the small number of cases in the study, a meaningful conclusion regarding the efficacy of deferasirox in this setting cannot yet be drawn. The most common adverse events of deferasirox therapy are gastrointestinal events and nephrotoxicity. Considering that nephrotoxic drugs, such as calcineurin inhibitors, are frequently used in HCT recipients, the safety of deferasirox administration in this setting requires further evaluation.3
In summary, the management of patients with high serum ferritin levels after HCT requires knowledge of the underlying pathophysiological mechanisms for hyperferritinemia; specifically, whether it is secondary to iron overload. Once iron overload has been proven, it is logical to start an iron-depleting treatment. However, more studies are required to assess the long-term deleterious effect of iron excess in this patient population and to define the best therapeutic strategy in this setting. Accordingly, enrollment in clinical trials should be encouraged. It is hoped that the information from the current analysis will instigate further studies to evaluate the role of iron overload after HCT and to develop strategies to prevent its long-term sequelae.
Conflict-of-interest disclosure: The author declares no competing financial interests.