In this issue of Blood, Walter et al provide an x-ray crystallographic structure of the factor VIII C2 domain in complex with 2 antibodies that illuminates how inhibitory antibodies complicate hemophilia A therapy.1
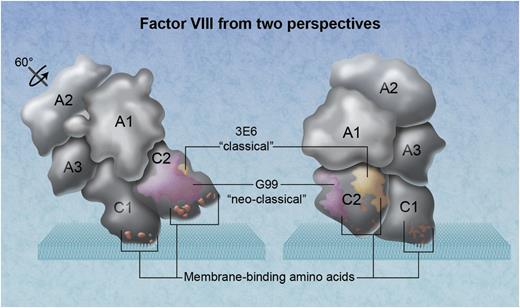
The 3-dimensional arrangement of factor VIII domains in relationship to a phospholipid membrane. The factor VIII molecule on the left has been rotated ∼60° back from its membrane-binding angle. Amino acid clusters that participate in membrane binding, demonstrated by mutagenesis experiments, are orange. The location of the neoclassical antibody G99 is depicted as magenta, identifying a thrombin and factor Xa interactive site. The epitope for the classical inhibitory antibody 3E6 is yellow. Professional illustration by Alice Y. Chen.
The 3-dimensional arrangement of factor VIII domains in relationship to a phospholipid membrane. The factor VIII molecule on the left has been rotated ∼60° back from its membrane-binding angle. Amino acid clusters that participate in membrane binding, demonstrated by mutagenesis experiments, are orange. The location of the neoclassical antibody G99 is depicted as magenta, identifying a thrombin and factor Xa interactive site. The epitope for the classical inhibitory antibody 3E6 is yellow. Professional illustration by Alice Y. Chen.
Inhibitory antibodies against factor VIII thwart effective treatment of hemophilia A. Nearly half target the C2 domain, underscoring the importance of its interactions. Mapping of antibody epitopes is a fruitful approach to understanding function of factor VIII and strategies to avoid hindrance by inhibitory antibodies.
Only membrane-bound, activated factor VIII can form an efficient complex with factor IXa to produce factor Xa and quench bleeding. The C2 domain of factor VIII participates in membrane binding. Factor VIII also binds to von Willlebrand factor (vWf) via the same surfaces that bind membranes. Antibodies that block binding to vWf and to membranes are dubbed “classical” inhibitors.
The C2 domain also contains a thrombin and factor Xa recognition motif, a factor IXa contact site, and possibly a factor Xa contact site. Inhibitory antibodies that prevent activation by thrombin or factor Xa or assembly with factor IXa and factor X are dubbed “nonclassical” inhibitory antibodies. Walter et al report the radiograph crystallographic structure of the factor VIII C2 domain in complex with the Fab fragments of mAb 3E6, a classical inhibitor, and mAb G99, a nonclassical inhibitor.1
Recent biochemical studies indicate that function of the C2 domain is more complicated than expected. For example, the isolated C2 domain does not compete with factor VIII for membrane binding and does not interfere with the factor VIIIa–factor IXa complex.2 Furthermore, factor VIII engineered to lack the C2 domain, retains functional activity, although with a requirement for a higher concentration of phospholipid vesicles with a high density of negative charge.3 In short, recent biochemical studies suggest that the C2 domain could have only marginal importance. It is the clinical anti-C2 antibodies that remind us that the function of the C2 domain is critically important and may provide insight that reconciles biochemical data with clinical facts.
The structure reported by Walter et al identifies the epitope recognized by thrombin or factor Xa. It provides more insight into the surface that contacts vWf. In addition, there are 2 surprises. First, the epitope of 3E6 does not include any of the 3 clusters of membrane-interactive amino acids established by site-directed mutagenesis and x-ray crystallography (see figure).4-7 Second, the epitope of 3E6 is not on, or adjacent to, the plane of factor VIII that contacts a membrane.6 Thus, the structure poses an enigma for factor VIII biochemistry.
Could it be that the contribution of the 3E6 epitope is a long distance charge interaction that is blunted by 3E6? Is it possible that the C2 domain makes a rolling motion as it contacts the membrane and the 3E6 is a contact site rather than binding surface? Does the flexibility of the C2 domain contact enable it to assume several possible orientations toward the membrane? This crystal structure motivates these questions and provides constraints around plausible answers. The answer to these questions is intriguing from a biochemical perspective and may also have importance in the design of engineered factor VIII therapeutics that are less susceptible to inhibitory antibodies.
Conflict-of-interest disclosure: The author declares no competing financial interests.