Abstract
Imatinib combined with chemotherapy for induction and intensification therapy has become the standard strategy for Philadelphia-positive acute lymphocytic leukemia (Ph+ALL). Anyhow, intensified chemotherapy lead to about 5% of early death. Because we believed that imatinib plays the most important role in Ph+ALL treatment, we started Ph+ALL-HX-200803 trial (WHO ICTRP Registry No. ChiCTR-TNRC-00000309 ) to test the effect of combining imatinib and low dose chemotherapy and added interferon-¦Á in maintenance to prevent relapse.
According to our protocol, all patients received imatinib 400mg daily, vindesine 4 mg weekly and dexamethasone 10mg/m2/day for 4 days per week for 4 weeks as induction therapy. For patients under 55 years old, we give them three sequential courses of intensified chemotherapy (CAM, high dose MTX+L-Asp, and MA, CALLG2008 protocol). Patients in CR1 would receive allogenic HSCT if they had suitable donors. Those who were reluctant to receive or unsuitable for allo-HSCT received maintenance therapy with imatinib 400mg daily, interferon-¦Á 3 million unit 2-3 doses per week and chemotherapy. Maintenance chemotherapy including vindesine and dexamethasone was given monthly in the first year, once every two months in the second year, and once every three months in the third year. For patients over 55 years old, we skipped intensified chemotherapy and gave them maintenance therapy directly. Minimal residual disease surveillance was conducted by BCR/ABL fusion gene quantification. The main endpoints were 3-year overall survival and disease free survival.
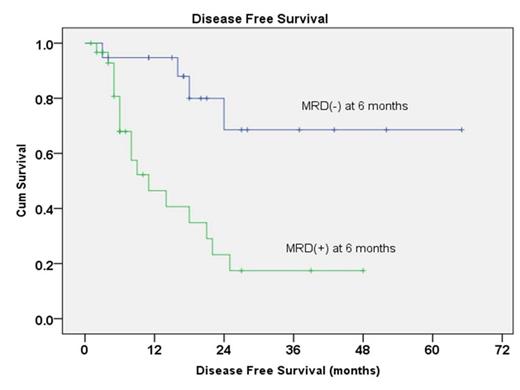
Between 2008 and 2012, 50 patients with newly diagnosed Ph+ALL were enrolled. The median age of this group of patients was 35.5 years old. All but one patients achieved complete remission (98%) after 4 weeks induction therapy. No patient died during induction therapy. The median follow-up time was 27 months. The estimated 3-year DFS and OS were 38.8 ±9.2% and 52.7±10.4%. Five patients received allo-HSCT in CR1. For patients who did not receive allo-HSCT in CR1, 1 patient survived for more than 5 years, 7 patients survived for more than 3 years. In post-hoc analysis, patients achieved MRD negativity at six month showed better median DFS (not reached vs. 11 moths, p=0.001) and OS (not reached vs. 22 months, p=0.015) compared to those who did not.
The outcomes of our study suggest that imatinib combing with low dose chemotherapy showed a high and safe induction remission rate, combination of interferon-¦Á with imatinib and maintenance chemotherapy might improve the outcomes of patients with Ph+All who were not eligible for Allo-HSCT. MRD status at six month is an important prognostic indicator. The long term disease-free survivors may signal the possibility of a cure even without allo-HSCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.