Abstract
Despite major advances in treatment, resistance and intolerance (R/I) continue to be a significant challenge in the management of CP-CML, with failure of commonly used 2G TKIs (dasatinib, nilotinib, bosutinib) occurring in >50% of patients over the course of treatment in the post-imatinib setting. Treatment options for patients who developed R/I to these 2G TKIs often had been limited to stem cell transplant or clinical trials. The availability of the new pharmacologic treatments has provided more options for patients in this setting. Ponatinib is the only TKI recommended to treat all TKI-resistant mutations listed in the National Comprehensive Cancer Network (NCCN) clinical practice guidelines, including the T315I mutation for which no other TKI is effective. However, the comparative efficacy of using ponatinib versus switching to a different pharmacologic treatment after 2G TKI failure has not been systematically examined.
A systematic review was conducted in MEDLINE, EMBASE and the Cochrane Libraries (2002-2012) as well as 3 conferences (ASH, ASCO, and EHA) over 5 years (2008-2012). Studies were included if they enrolled 10 or more adult patients and presented results for CP-CML patients who were R/I following 2G TKI treatment. All study designs were considered and no restriction was applied with respect to therapy dose, due to incomplete reporting of doses in the available studies.
Reported cumulative response rates (regardless of time period of reporting) and derived confidence intervals from individual studies were plotted, with node size determined by number of patients in study arm. In addition, Bayesian methods were used to synthesize major cytogenetic response (MCyR) and complete cytogenetic response (CCyR) from individual studies and estimate the overall response probability with 95% credible interval (CrI) for each treatment. Bayesian analysis also was used to estimate the likelihood that each treatment offers the highest probability of CCyR/MCyR based on available evidence. Response probabilities for ponatinib were estimated using individual patient data from the phase 2 PACE study and published phase 1 study data. For the PACE study, only patients who had received 2 prior TKIs were included in the analysis, to provide an appropriate comparison to the published 2G TKI studies which included predominantly 3rd line patients.
The base case analysis includes patients both with and without the T315I mutation; a sensitivity analysis focused on the subset of studies without substantial numbers of enrollees with the T315I mutation and those that presented subgroup results excluding patients with the mutation.
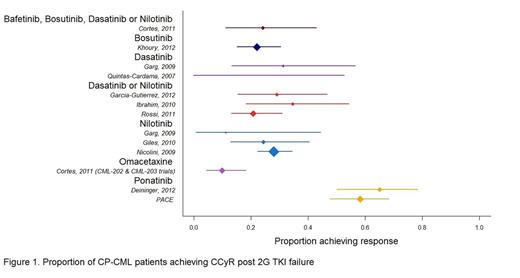
13 clinical trials and observational studies were included in the analysis. All studies reported CCyR and 8 reported MCyR. CCyR data from each study, with derived confidence intervals, are presented in Figure 1.
Synthesized treatment-specific probabilities of CCyR across 2G TKIs ranged from 22.0% (bosutinib) to 26.9% (nilotinib) compared to 60.5% (95% CrI 52.1% to 68.6%) for ponatinib. Treatment-specific probabilities of MCyR across 2G TKIs ranged from 29.7% (bosutinib) to 49.9% (dasatinib or nilotinib) compared to 70.2% (62.1% to 77.7%) for ponatinib. CCyR and MCyR rates for omacetaxine were lower than those observed for both the 2G TKIs and ponatinib (9.9% and 19.8% respectively). The probability of ponatinib providing superior response to all other included treatments was estimated at 99.9% for CCyR and 97.4% for MCyR. Results in patients without the T315I mutation were similar: the probability of CCyR with ponatinib was 52.1% (41.9% to 62.4%) compared to 22.1% to 26.9% across 2G TKIs. The probability of MCyR with ponatinib was 64.2% (54.1% to 73.6%) compared to 29.7% to 50.0% across 2G TKIs.
The analysis suggests that CP-CML patients who are resistant or intolerant to prior 2G TKI therapies have higher complete (33.6 to 38.5 percentage points higher) and major (20.3 to 40.5 percentage points higher) cytogenic response with ponatinib compared with the 2G TKIs included in this analysis. Based on available data, ponatinib appears to provide a higher probability of treatment response compared to all other pharmacologic treatments commonly used in this indication. This observation was consistent both for the overall patient population and among the subset of patients without the T315I mutation.
Lipton:Novartis: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol Myers Squibb: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding, Speakers Bureau; Ariad: Equity Ownership, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding, Speakers Bureau. Bryden:Ariad Pharmaceuticals, Inc.: Research Funding. Sidhu:Ariad Pharmaceuticals, Inc.: Research Funding. Huang:Ariad Pharmaceuticals, Inc.: Employment. McGarry:Ariad Pharmaceuticals, Inc.: Employment; GSK: Consultancy; BMS: Consultancy; Pfizer: Consultancy; Janssen (J&J): Consultancy. Lustgarten:ARIAD: employees of and own stock/stock options in ARIAD Pharmaceuticals, Inc Other, Employment. Woods:Ariad Pharmaceuticals, Inc.: Research Funding. Hawkins:Ariad Pharmaceuticals, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.