Abstract
Thrombocytopenia is a common (40-65%) manifestation of MDS. The incidence of bleeding ranges from 3% to 56% encompassing minor bleeds such as petechiae and gingival bleeding, and more serious bleeds (18%), such as gastrointestinal (GI, 6-7%) and intracranial (3-5%) bleeding. Lenalidomide and azacitidine are not specifically used or approved for the treatment of thrombocytopenia in MDS and while effective in some patients, may in fact engender a thrombocytopenia that is dose limiting. The interaction between thrombopoietin (TPO) and its receptor c-Mpl is essential for platelet production. Romiplostim and Eltrombopag are two TPO-receptor agonists presently approved by the FDA. They have been tested in MDS, with some evidence of benefit, but there are safety concerns regarding progression to acute myeloid leukemia (AML).
To determine the safety, as well as the clinical outcomes of adding a TPO-receptor agonist to standard treatment in patients with myelodysplastic syndromes.
A systematic literature search strategy was conducted up to February 2013, using MEDLINE, EMBASE, the Cochrane Register of Controlled Trials, as well as conference proceedings. All randomized controlled trials (RCTs) that enrolled adult patients with myelodysplastic syndromes were included if the RCT compared a TPO-R agonist (Romiplostim or Eltrombopag) to placebo or no treatment. A meta-analysis of the effects was performed. Pooled treatment effects were calculated as risk ratios (RR) for binary data, using a random effects model. Bleeding and platelet transfusion rates were also reported as exposure-adjusted rates per patient-month, as it was felt to be a meaningful time period for this disease. The exposure adjusted rate was defined as the number of events per person-time at risk and captures the total cumulative bleeding events (even multiple events within the same patient). Months of person time were defined as months from first dose date to study end date.
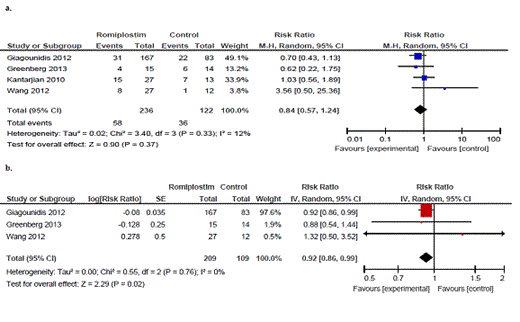
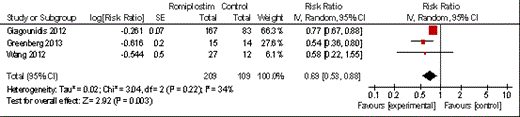
Four RCTs met all inclusion criteria, and a total of 358 patients were included in the systematic review. 2 studies included only lower risk MDS patients (IPSS low/int-1) and 2 studies included lower and higher risk patients (IPSS low/int-1 and int-2). Romiplostim was compared to placebo in all 4 trials, but each trial had a different backbone for MDS therapy (lenalidomide, azacitidine, decitabine and just placebo). Two trials compared 2 dosing regimens to placebo (500ug weekly and 750ug weekly) and 2 trials used the higher dosing of 750ug weekly. Three studies of eltrombopag vs. placebo are ongoing, and results were not available for inclusion. Relative risk (RR) of bleeding with romiplostim vs. placebo was 0.84 (95% CI: 0.57-1.24; I2:12%). Exposure-adjusted bleeding rate per patient month was significantly less with romiplostim vs. placebo at 0.92 (95% CI: 0.86-0.99; I2:0%). Only one study reported severe grade 3-4 bleeding events, and the rates were very low, at 1/27 in the intervention arms (4%) and 2/13 (13%) in the control group, giving a favourable RR of 0.24, but very wide 95% CI of 0.02 to 2.42. The exposure-adjusted platelet transfusion rate per patient month was also significantly less with romiplostim at 0.69 (95% CI: 0.53-0.88; I2:34%). The RR of AML progression with romiplostim vs. placebo was 1.36 (95% CI: 0.54-3.40; I2:0%), however the AML progression outcome data were felt to be at high risk of bias because of the early termination of one trial. Mortality was reported as a proportion of deaths during the study period for all 4 trials, giving a RR with romiplostim of 0.90 (95% CI: 0.54-1.50; I2: 30%).
Romiplostim is promising in its ability to decrease patient-important outcomes: cumulative bleeding events and the need for platelet transfusions. However, most of those bleeding events were likely grade 2, which are not necessarily clinically important, and platelet transfusion reduction may not be as relevant for those practicing therapeutic (not prophylactic) platelet transfusions. Although the risk of AML progression was not increased, the outcome data were felt to be at high risk for bias, and thus this safety concern cannot be ignored. Therefore, romiplostim cannot yet be routinely recommended. Results of ongoing eltrombopag studies are awaited.
a. Total Bleeding Events b. Exposure-adjusted bleeding rate
a. Total Bleeding Events b. Exposure-adjusted bleeding rate
Exposure-adjusted platelet transfusion rate
AML progression rate
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract