Abstract
In the context of redirected T-cell based antitumor adoptive immunotherapy, the therapeutic roles played by co-infused CD4+ T cells genetically redirected to the predefined HLA class I-restricted epitope which had been originally recognized by effector CD8+ T cells has not yet been fully discussed. In this study, using an HLA class I-restricted WT1 -specific T-cell receptor (TCR) gene transfer, we in detail examined antileukemia functionality mediated by these gene-modified CD4+ T cells co-infused with similarly gene-modified effector CD8+ T cells as the redirected T cell-based adoptive immunotherapy.
Using our unique retroviral vector expressing HLA-A*2402-restricted and WT1235-243-specific TCR a/b genes and shRNAs for endogenous TCRs (WT1-siTCR vector), we genetically modified both CD4+ and CD8+ T cells from the same healthy donor or leukemia patients (termed WT1-siTCR/CD4 and WT1-siTCR/CD8, respectively). First, target-responsive cellular outputs mediated by WT1-siTCR/CD4 was thoroughly examined using flowcytometry, ELISA, 51Cr-release assay, CFSE dilution assay and bioluminescence assay. Next we similarly assessed impacts of WT1-siTCR/CD4 on the antileukemia functionality mediated by concurrentWT1-siTCR/CD8 both in vitro and in vivo. Eventually, we assessed the in vivo therapeutic efficacy of combined administration of WT1-siTCR/CD8 with WT1-siTCR/CD4 using a xenografted mouse model.
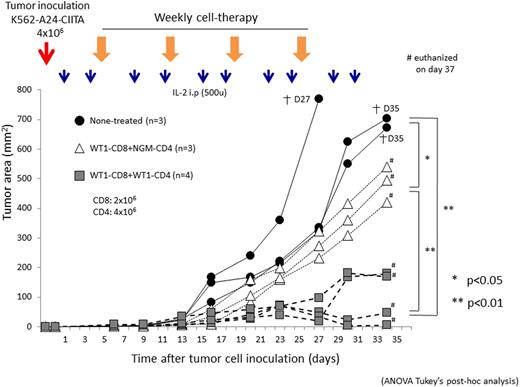
The transcription factor profile demonstrated that WT1-siTCR/CD4 turned a terminal effector, but not regulatory phenotype. Activated WT1-siTCR/CD4 expressed cell-surface CD40L. Target-responsive cytokine production profile of WT1-siTCR/CD4 represented the Th1 helper function in the context of HLA-A*2402. HLA class II molecules expressed by leukemia cells facilitated the recognition of leukemia cells by WT1-siTCR/CD4 in the context of HLA-A*2402. WT1-siTCR/CD4 displayed the delayed cytocidal activity determined by 51Cr release assay. WT1-siTCR/CD4 could produce IFN-g in response to freshly isolated leukemia cells. WT1-siTCR/CD4 displayed the leukemia trafficking activity in vivo. WT1-siTCR/CD4 represented the potential to migrate into bone marrow via CXCR4/CXCL12 axis both in vitro and in vivo. Concurrent WT1-siTCR/CD4 augmented IFN-g production and cytotoxic degranulation mediated by WT1-siTCR/CD8 in response to the cognate epitope via humoral factors. Consequently, the cytocidal activity against autologous leukemia cells mediated by WT1-siTCR/CD8 was augmented in the presence of WT1-siTCR/CD4, both of them generated from normal lymphocytes of the same patient with leukemia in a complete remission. Upon the target recognition, activated WT1-siTCR/CD4 recruited WT1-siTCR/CD8 via CCL3/4-CCR5 axis. Proliferative response and differentiation into central memory T-cell subset mediated by WT1-siTCR/CD8 in response to the cognate epitope and leukemia cells were enhanced in the presence of autologousWT1-siTCR/CD4, but not gene-modified CD4+ T cells (NGM-CD4). CD127 expression on activated WT1-siTCR/CD8 also increased in parallel to this differentiation. Co-infused WT1-siTCR/CD4 augmented the tumor trafficking and persistence of WT1-siTCR/CD8 in vivo, resulting in the greater suppression of leukemia cells in a xenografted mouse model. Finally, in the therapeutic mouse model, co-infusion of WT1-siTCR/CD8 with of WT1-siTCR/CD4 significantly suppressed the growth of inoculated leukemia cells compared to that in mice received co-infusion of WT1-siTCR/CD8 with NGM-CD4 (Fig.1). Correlation between the therapeutic efficacy and survival of infused gene-modified T cells was also observed.
In results, the combined infusion of WT1-siTCR/CD8 with WT1-siTCR/CD4, but not NGM-CD4 obviously demonstrates the enhanced antileukemia efficacy via diverse mechanisms. Now we have just started a clinical trial using gene-modified T cells with WT1-siTCR vector for the treatment of patients with refractory acute myeloid leukemia and myeloid dysplastic syndrome. Because redirected T cells employed in this trial encompassed both WT1-siTCR/CD4 and WT1-siTCR/CD8, we are planning to clinically verify the significance of WT1-siTCR/CD4 in the redirected T cell-based antileukemia adoptive immunotherapy.
(Fig.1)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.