In this issue of Blood, Jaax and colleagues show that heparin-PF4 antibodies cross-reacted with nucleic acid (NA)–PF4 complexes and induced platelet activation, suggesting that NA-PF4 can potentially cause a heparin-induced thrombocytopenia (HIT)–like prothrombotic disorder.1
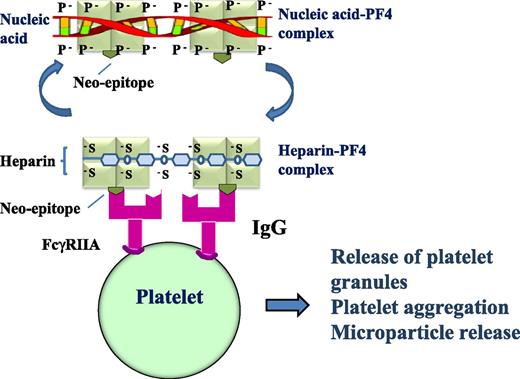
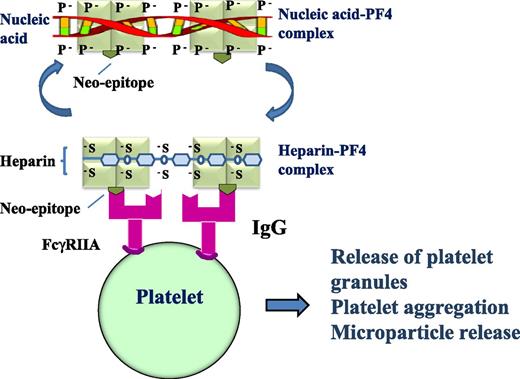
Heparin-PF4 antibodies can cross-react with NA-PF4 and induce platelet activation. IgG heparin-PF4 antibodies react with heparin-PF4 complex or NA-PF4 complex, the antigen-IgG complex then binds to and cross-links platelet FcγRIIA receptors resulting in platelet activation with granule and microparticle release, platelet aggregation, and thrombus formation. The light green square indicates PF4; the olive green inverted symbol represents the neo-epitope that emerges after PF4 undergoes a conformational change after heparin or nucleic acid binds PF4; and the magenta symbol indicates that the heparin-PF4 IgG antibody cross-reacts with nucleic acid-PF4.
Heparin-PF4 antibodies can cross-react with NA-PF4 and induce platelet activation. IgG heparin-PF4 antibodies react with heparin-PF4 complex or NA-PF4 complex, the antigen-IgG complex then binds to and cross-links platelet FcγRIIA receptors resulting in platelet activation with granule and microparticle release, platelet aggregation, and thrombus formation. The light green square indicates PF4; the olive green inverted symbol represents the neo-epitope that emerges after PF4 undergoes a conformational change after heparin or nucleic acid binds PF4; and the magenta symbol indicates that the heparin-PF4 IgG antibody cross-reacts with nucleic acid-PF4.
HIT is a serious prothrombotic complication of heparin treatment.2,3 Approximately 50% of patients who develop HIT suffer thrombosis that is often severe and extensive.2,3 Even with treatment using a nonheparin anticoagulant (such as argatroban), death and leg amputation (due to limb gangrene) still occur in 23% and 15% of patients, respectively.4 HIT is mediated by immunoglobulin G (IgG) heparin-PF4 antibodies. Heparin-PF4-IgG complexes bind and cross-link platelet FcγIIA receptors inducing platelet activation, granule and microparticle release, platelet aggregation, and thrombus formation.3 For many years, clinicians have been puzzled by several unexplained observations (see figure). First, HIT occurs more frequently in patients with tissue damage, which can occur after a major surgery, major trauma, or severe infection.5 Second, a HIT-like thrombotic disorder (termed “spontaneous HIT”) develops in patients who never received heparin administration.6 Finally, patients with “delayed-onset HIT” have thrombocytopenia and thrombosis (often severe) that can occur after cessation of heparin and persist without further heparin administration.7 These observations suggest that in addition to heparin, there may be another negatively charged compound that forms an immunogenic complex with the positively charged PF4.
Jaax et al hypothesized that the negatively charged compound is NA, and it could substitute heparin in the heparin-PF4-IgG complex that induces platelet activation.1 They were able to show that NAs (including aptamers) could form complexes with PF4 and that longer or double-stranded NAs were better at complex formation. They also found that upon NA binding, PF4 underwent a conformational change similar to that induced by heparin. As expected, human heparin-PF4 antibodies cross-reacted with NA-PF4 complexes, and the antigen-IgG complexes were capable of initiating platelet activation (see figure). Furthermore, when mice were immunized with murine PF4-NA complexes, the PF4-NA antibodies generated cross-reacted with murine PF4-heparin complexes. These findings may provide explanations for the unexplained observations in patients with HIT and HIT-like disorder.
Recent studies have reported that NAs (including DNA and RNA) are present in human plasma in detectable amounts. Although they are found in low concentrations in healthy individuals, NA plasma levels are significantly raised in some pathological conditions8 such as infection, postsurgical state, multiple trauma, and cancer. Presumably, NAs are released into the circulation after breakdown of human cells, bacteria, or virus. In these conditions, platelet activation and PF4 release from platelets would result in increased plasma PF4 levels and these coincide with an increase in plasma DNA/RNA concentrations (due to breakdown of human cells or microorganisms). Any NA-PF4 complexes formed may induce an immunologic response and generation of NA-PF4 antibodies that would cross-react with heparin-PF4 complexes. Importantly, NAs are eliminated very rapidly by plasma nuclease degradation and renal excretion. This may reduce, although probably not prevent, immunization because heparin-PF4 from a single low dose of heparin is known to induce development of HIT.
If this is true, the findings of Jaax and colleagues could explain the above previously unexplained observations regarding HIT. First, raised plasma NA-PF4 levels during surgery would prime patients to the development of HIT if heparin is given postoperatively. This could explain the increased frequency of HIT in patients undergoing major surgery such as hip/knee replacement.5 Second, patients reported to have “spontaneous HIT” usually have serious infections or have undergone major orthopedic surgery.6 In these patients, elevated plasma NAs from tissue or bacterial breakdown would substitute for heparin in the immune reactions even though these patients never received heparin. Third, again NAs could take the place of heparin in patients with “delayed-onset HIT.”7 In these cases, thrombocytopenia and thrombosis occur and persist after cessation of heparin.7 This may be due to continued NA release in the bloodstream from ongoing tissue damage and cell breakdown.
The findings of these investigators also have other clinical implications. Use of therapeutic oligonucleotides is a promising therapeutic strategy that is being investigated in clinical trials and is starting to be used in clinical practice. For example, pegaptanib is an RNA aptamer approved for use as an anti–vascular endothelial growth factor agent.9 These aptamers can be antigenic and some have a long plasma half-life. The results of Jaax et al provide a timely warning of a potential HIT-like complication with the use of these agents. Patients receiving these new agents should be carefully monitored for the development of a HIT-like complication. Beyond drug side-effects, the study of Jaax et al may have much wider-reaching implications. The development of NA-PF4 antibodies and platelet activation might impose a thrombocytopenia and thrombosis risk that has been previously unknown. This is because elevated levels of RNA/DNA are present in the blood of many patients including those with infection or proinflammatory/malignant conditions, after major surgery and multiple traumas. If this increased risk is confirmed by further studies, NA-PF4 antibodies could serve as an independent thrombosis risk factor similar to the current use of anti-phospholipid antibodies.
In conclusion, although the data by Jaax et al1 are novel, stimulating, and thought-provoking, they must be considered as preliminary. Obviously, further studies with confirming data are needed before the pathogenic role of NAs is fully accepted. Additionally, the significance of in vitro platelet activation, shown by Jaax et al, would be strengthened by the development of an animal model demonstrating in vivo thrombocytopenia and thrombosis induced by NA-PF4 antibodies in the presence of NA and PF4. Further investigations of the neo-epitopes that emerge upon heparin vs NA binding to PF4 and more detailed characterization of heparin-PF4 vs NA-PF4 antibodies will also provide further insights into the pathogenesis of “spontaneous HIT” and “delayed-onset HIT” if NAs play a role in the pathophysiology of these disorders.
Conflict-of-interest disclosure: The authors declare no competing financial interests.