In this issue of Blood, Casari et al demonstrate that mutant platelet survival is shorter and clearance of mutant von Willebrand factor (VWF)–platelet complexes increased in a mouse model of type 2B von Willebrand disease (VWD2B) compared with control mice, providing a comprehensive explanation for thrombocytopenia in VWD2B.1
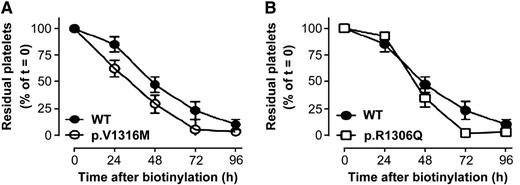
Platelet life span is reduced in VWD2B mice with respect to WT-mVWF–expressing mice. Mice expressing WT-mVWF (A-B, closed circles), p.V1316M-mVWF (A, open circles), or p.R1306Q-mVWF (B, open squares) were infused with NHS-biotin, which allows platelet biotinylation. Residual biotinylated platelets were quantified by flow cytometry. Data are expressed as the percentage of biotinylated platelets relative to the total CD41-positive platelet population with t = 0 being arbitrarily set at 100%. Data represent mean ± standard deviation. N = 3 to 13 mice. See Figure 7 in the article by Casari et al that begins on page 2893.
Platelet life span is reduced in VWD2B mice with respect to WT-mVWF–expressing mice. Mice expressing WT-mVWF (A-B, closed circles), p.V1316M-mVWF (A, open circles), or p.R1306Q-mVWF (B, open squares) were infused with NHS-biotin, which allows platelet biotinylation. Residual biotinylated platelets were quantified by flow cytometry. Data are expressed as the percentage of biotinylated platelets relative to the total CD41-positive platelet population with t = 0 being arbitrarily set at 100%. Data represent mean ± standard deviation. N = 3 to 13 mice. See Figure 7 in the article by Casari et al that begins on page 2893.
In 1980, Ruggeri et al reported a group of patients with VWD presenting a puzzling and unique feature.2 Although plasma VWF was variably reduced, in vitro ristocetin-induced platelet agglutination occurred at concentrations lower than those required for normal controls and other patients with VWD. This was suggested to be the result of paradoxical heightened interaction of the abnormal VWF with the physiological glycoprotein Ibα (GpIbα) receptor on the platelet membrane.2 They also observed variable lack of high-molecular-weight multimers of VWF and named the disorder type IIB (now 2B) VWD to differentiate it from another qualitative variant, type IIA, in which very high concentrations of ristocetin were needed to agglutinate patients’ platelet-rich plasma and multimer abnormalities were more profound. A subsequent study by Holmberg et al3 showed that in VWD2B patients the administration of desmopressin could induce a variable degree of thrombocytopenia, and it soon became evident that a significant proportion of patients with VWD2B may have chronic thrombocytopenia as a result of this heightened binding of mutant VWF (mVWF) to the platelets.4 Now we know that VWD2B is a rare (∼5% of all VWD cases), usually highly penetrant autosomally inherited bleeding disorder caused by gain-of-function mutations in the A1 domain of VWF.4 Approximately 50% of patients with this type exhibit mild to moderate thrombocytopenia, which can be unraveled or further aggravated by some clinical circumstances (eg, surgery, infection, or pregnancy) because of the increased release of the abnormal VWF by the endothelial cells.4 However, although patients with some mutations are particularly prone to this phenomenon (eg, I1309V and V1316M), in others no thrombocytopenia episodes occur (eg, P1266Q/L or R1308L).4 Thrombocytopenia inversely correlates with the increase of circulating levels of the conformationally active form of type 2B VWF, which may also interact spontaneously with GpIbα.4 From a clinical point of view, the risk of bleeding is approximately fivefold higher in VWD2B patients with associated thrombocytopenia below 140 × 103 platelets per μL compared with those with normal platelet counts.4
Apart from affecting the interaction with GpIbα, recent findings suggest that type 2B VWF may also affect platelet production. In addition to platelet aggregates, giant platelets may also be evident in peripheral blood smears of VWD2B patients.4 Megakaryocytes from patients with VWD2B have an altered morphology and produce abnormal and fewer platelets than normal controls, suggesting that the continuous interaction with GpIbα of abnormal VWF during megakaryocyte maturation influences the formation of platelets in these patients.5 However, it is still unclear whether VWD2B platelets have normal survival in circulation. Furthermore, it has been hypothesized that thrombocytopenia is also caused by enhanced clearance of the abnormal VWF-platelet complexes, but no experimental proof has been provided until now.
The study by Casari and colleagues is aimed at filling these gaps.1 They developed a murine model for VWD2B that reproduces the phenotype observed in human patients with the disorder. As an important background to the study, the same group previously reported that macrophages in liver and spleen bind and endocytose VWF and that chemical blockade of these cells leads to a twofold prolongation of VWF half-life in circulation.6 Furthermore, it was recently found that activation of VWF via exposure to shear stress enhances macrophage uptake, thus contributing significantly to the clearance of VWF.7
Casari and colleagues1 found that murine VWD2B platelets, depending on the type of mutation, have a variably shorter circulatory survival than wild-type (WT) platelets, which could contribute to the lower platelet count in VWD2B mice (see figure). This underlines the role of type 2B VWF in inducing the formation of abnormal platelets with shortened life span in circulation. Importantly, further analysis revealed that type 2B VWF is exclusively present at the surface of platelets of thrombocytopenic VWD2B mice, suggesting that VWF binding to platelets is needed to induce thrombocytopenia. These VWF-platelet complexes are taken up efficiently by macrophages in liver and spleen, thus accelerating their clearance. Interestingly, increase of circulating levels of the conformationally active form of type 2B VWF also promotes VWF binding by macrophages. The presence of increased levels of conformationally active VWF has also been demonstrated in other acquired conditions with thrombocytopenia (eg, thrombotic thrombocytopenic purpura) with increased uptake of platelets by macrophages.8 This could be a protective mechanism to remove VWF-platelet complexes, preventing the risk of occlusion of microvasculature in VWD2B. Macrophage depletion leads to a two- to threefold increase of platelet counts in thrombocytopenic mice with the V1316M mutation, characterized by the most severe thrombocytopenia. Macrophage depletion has also been demonstrated to determine platelet increase in a mouse model for immune thrombocytopenia,9 confirming the importance of these cells in the clearance mechanisms of platelet-containing complexes.
Thus, thrombocytopenia in VWD2B appears to be the result of at least a combination of shortened survival of the abnormal platelets and of an accelerated clearance of the abnormal VWF-platelet complexes by macrophages in liver and spleen. VWD2B appears to be not only a disorder of a plasma hemostatic protein but also of platelets, suggesting that in addition to VWF/factor VIII concentrates, transfusion of normal platelets may be justified in VWD2B patients with bleeding and worsening of their thrombocytopenia due to hemostatic stress situations.
Because increased clearance of mVWFs appears to be more frequent than expected in VWD,10 the model developed by Casari and colleagues could be used to investigate whether mVWFs with accelerated clearance have a preferentially increased uptake by macrophages through enhanced binding to special receptors (eg, low-density lipoprotein receptor–related protein 1, LRP1), thus highlighting the role of specific VWF regions critical in this regard.
Conflict-of-interest disclosure: The author declares no competing financial interests.