Key Points
Carbon monoxide treatment of murine sickle mice can ameliorate inflammation and vaso-occlusion.
MP4CO induces heme oxygenase-1 and Nrf2 to mediate these salutatory effects.
Abstract
Transgenic sickle mice expressing βS hemoglobin have activated vascular endothelium in multiple organs that exhibits enhanced expression of NF-ĸB and adhesion molecules and promotes microvascular stasis in sickle, but not normal, mice in response to hypoxia/reoxygenation (H/R), or heme. Induction of heme oxygenase-1 (HO-1) or administration of its products, carbon monoxide (CO) or biliverdin, inhibits microvascular stasis in sickle mice. Infusion of human hemoglobin conjugated with polyethylene glycol and saturated with CO (MP4CO) markedly induced hepatic HO-1 activity and inhibited NF-ĸB activation and H/R-induced microvascular stasis in sickle mice. These effects were mediated by CO; saline or MP4 saturated with O2 (MP4OX) had little to no effect on H/R-induced stasis, though unmodified oxyhemoglobin exacerbated stasis. The HO-1 inhibitor, tin protoporphyrin, blocked MP4CO protection, consistent with HO-1 involvement in the protection afforded by MP4CO. MP4CO also induced nuclear factor-erythroid 2 p45-related factor 2 (Nrf2), an important transcriptional regulator of HO-1 and other antioxidant genes. In a heterozygous (hemoglobin-AS) sickle mouse model, intravenous hemin induced cardiovascular collapse and mortality within 120 minutes, which was significantly reduced by MP4CO, but not MP4OX. These data demonstrate that MP4CO induces cytoprotective Nrf2 and HO-1 and decreases NF-ĸB activation, microvascular stasis, and mortality in transgenic sickle mouse models.
Introduction
Heme oxygenase-1 (HO-1) is a cytoprotective enzyme that degrades free heme to carbon monoxide (CO), biliverdin, and iron. Induction of HO-1 activity elicits protective effects in a variety of models of inflammatory, ischemic, and oxidant injury,1,2 and conversely, humans or mice lacking HO-1 are sensitized to oxidant injury.3,4 In transgenic sickle mice, HO-1 plays a vital role in the inhibition of endothelial activation, rolling and adhesion of leukocytes to the vessel wall and vaso-occlusion.5 The cytoprotective properties of HO-1 are mimicked by the products of the heme/HO-1 reaction, CO and biliverdin.5
CO is a potent mediator of cell protection and has a number of properties that make it an attractive therapeutic option for treating sickle cell disease (SCD) including vasodilator, anti-inflammatory, left-shift of the hemoglobin (Hb)–oxygen dissociation curve, upregulation of HO-1, and activation of cytoprotective cell signaling pathways.6,7 Beneficial effects of exogenous CO administration have been observed in SCD5,6 because CO may prevent HbS polymerization,8 Hb oxidation, and heme release9 and provide anti-inflammatory/antiadhesive actions.5 Additionally, CO induces HO-1 expression indirectly by its action on the stress-induced, antioxidant response element transcription factor nuclear factor-erythroid 2 p45-related factor 2 (Nrf2).10
The development of inhaled CO as a therapeutic agent is limited by questions of safety and the need for controlled delivery systems. MP4CO is a solution of polyethylene glycol (PEG)–conjugated human Hb saturated with CO gas. We have previously demonstrated that CO from MP4CO administered intravenously (IV) rapidly equilibrates with red cell Hb and exerts cytoprotective effects, reducing myocardial infarct size in rats.11 The objectives of the current research were to examine the potential benefits of CO by intravenous administration of MP4CO in transgenic sickle mouse models before and after a vaso-occlusive stimulus, hypoxia/reoxygenation (H/R). Our hypothesis was that the potential anti-inflammatory actions of MP4CO would diminish H/R-induced stasis in transgenic sickle mice. Additionally, we examined the mechanism of MP4CO action by comparing MP4CO with MP4 saturated with O2 (MP4OX) and the effects on NF-ĸB, Nrf2, and HO-1 expression.
Methods
Reagents
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless specified differently. MP4CO, MP4OX, and stroma-free Hb were formulated in lactated Ringer’s solution (LRS) and prepared by Sangart, Inc. (San Diego, CA), as described previously.12
Mice
All animal breeding and experiments were approved by the Institutional Animal Care and Use committees at the University of Minnesota Medical School and Sangart, Inc., and conducted in accordance with the Institute for Laboratory Animal Research Guide for Care and Use of Laboratory Animals. We used male and female NY1DD13 and heterozygous HbAS-Townes14 transgenic sickle mice at 8-13 weeks of age as models for human SCD and SCD trait, respectively. The NY1DD and HbAS mice are on C57BL/6 and mixed genetic backgrounds, respectively. The NY1DD mice are homozygous for deletion of the mouse βmajor globin locus and express a human α and βS globin transgene. Heterozygous HbAS mice were derived by breeding male HbSS-Townes mice (mα−/−; mβ−/−; hα+/+; hβS+/+) with female HbAS-Townes mice (mα−/−; mβ−/−; hα+/+; hβS+/−). Globin phenotypes in both strains were determined by isoelectric focusing.
Mouse treatments
Mice were treated with LRS, MP4CO, MP4OX, or unmodified Hb. MP4CO and MP4OX are 4.3 g/dL solutions of human Hb conjugated with PEG and saturated with CO or O2, respectively, and identical in all aspects (colloid osmotic pressure, molecular weight, concentration, production process) except the heme ligand, as previously published.11,12 Stroma-free Hb (without pegylation) is a 4.3-g/dL solution of human Hb saturated with O2. Mice with implanted dorsal skin-fold chambers (DSFCs) (see below) were given a bolus infusion via the tail vein of 2, 4, 8, or 12 mL/kg of LRS, MP4CO, MP4OX, or Hb 24 hours pre-H/R, 30 minutes post-H/R (1 hour hypoxia at 7% O2/93% N2, followed by 30 minutes in room air), or both. In some control mice, hemin chloride was injected intraperitoneally (IP) (40 µmoles/kg/day × 3 days) to induce HO-1, a known inhibitor of vascular stasis.5 Additionally, in some mice, tin protoporphyrin (SnPP) was injected IP (40 µmoles/kg/day × 3 days) to inhibit HO-1 activity.
Collection and measurement of exhaled CO
Volume of expired CO was measured in mice using a rebreathing collection technique and gas chromatography, as previously described.15
Vascular stasis in dorsal skinfold of NY1DD mice
H/R-induced stasis of venular blood flow in the subcutaneous skin was measured in NY1DD mice with implanted DSFCs using intravital microscopy as previously described 3 days after DSFC implantation.16 At baseline, with the mice in ambient air, flowing venules were selected at random, and their relative locations were mapped and recorded. The mice were then subjected to 1 hour hypoxia (7% O2/93% N2), followed by reoxygenation in room air. After 1 hour of reoxygenation, the same venules were reexamined for blood flow. Venules with no flow were counted as static, and the percentage of static venules were calculated. A minimum of 33 subcutaneous venules were examined in each mouse.
Mouse tissue collection
After 4 hours of reoxygenation, the mice were sacrificed and tissues harvested as described.16 Mice were asphyxiated in a CO2 chamber for approximately 2 minutes. Blood was collected by cardiac puncture for isolation of EDTA plasma. Livers were removed, frozen in liquid nitrogen, and stored at −85°C. Nuclear extracts and microsomes were prepared from livers as described.17
Measurement of HO enzyme activity
HO activity was measured in liver microsomes as described.17
Western blots of liver NF-ĸB phospho-p65, Nrf2, and HO-1
Liver nuclear extracts (NF-ĸB phospho-p65 and Nrf2) or microsomes (HO-1) were analyzed by western blot. The membranes were probed with rabbit anti-NF-ĸB phospho-p65 (Ser536) (Cell Signaling Technology, Beverly, MA), rabbit monoclonal anti-Nrf2 (Cell Signaling Technology) or mouse monoclonal anti–HO-1 (Enzo Life Sciences, Farmingdale, NY). Primary antibodies were visualized with alkaline phosphatase-conjugated goat anti-mouse or goat anti-rabbit IgG (Santa Cruz Biotechnology, Dallas, TX). Immunoreactive bands were visualized with ECF substrate (GE Healthcare, Pataskala, OH) and a Storm Reader (GE Healthcare). Microsomal HO-1 blots were stripped (Restore Stripping Buffer; Thermo Scientific, Waltham, MA) and reprobed with rabbit anti-GAPDH (Sigma-Aldrich).
Heme-induced cardiorespiratory collapse and mortality
Heterozygous HbAS-Townes mice were anesthetized with 2% isoflurane in 30% oxygen followed by maintenance with IP injection of a fentanyl (225 µg/kg)/ketamine (120 µg/kg) cocktail. Animals were tracheotomized and mechanically ventilated with 30% oxygen to maintain eucapnia (Kent Scientific, Torrington, CT). The right femoral artery and vein were cannulated for measurement of arterial pressure and introduction of drugs/fluids, respectively, after which supplemental oxygen was discontinued, and animals were ventilated with room air. Core temperature was maintained at 38°C. Arterial pressures were recorded at 100 Hz using a BIOPAC data acquisition system (BIOPAC Systems, Goleta, CA).
Following 10 minutes stabilization, hemin chloride (50 µmol/kg; Frontier Scientific, Logan, UT) was injected (0.3 mL bolus) IV. Ten minutes after hemin injection, mice were infused with 2, 4, or 8 mL/kg of saline, MP4OX, or MP4CO over 30 minutes. Immediately upon death or at 150 minutes posthemin, blood samples were taken by cardiac puncture.
Statistical analyses
All statistical analyses were performed with SigmaStat 2.0 for Windows (SPSS Inc., Chicago, IL) or JMP statistical software (SAS Institute, Cary, NC). Comparisons of vascular stasis in multiple treatment groups were made using 1-way analysis of variance with pairwise multiple comparisons made using the Holm-Sidak method. For the analysis of arterial pressure following hemin injection, differences between groups of animals were analyzed using a 2-way repeated-measures analysis of variance with treatment group and time as the 2 factors. Survival data were plotted as Kaplan-Meier graphs and analyzed by log-rank test.
Results
MP4CO rapidly releases CO in vivo
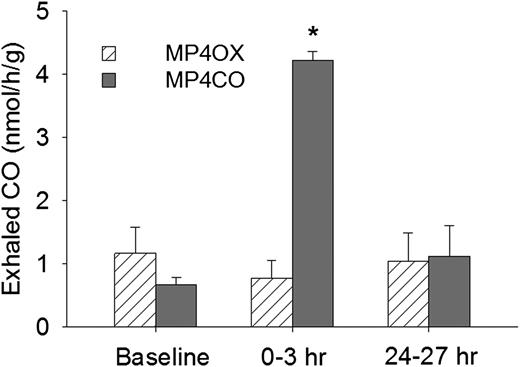
Exhaled CO was collected and measured 24 hours before infusion (baseline), 0-3 hours after infusion, and 24-27 hours after infusion of MP4CO or MP4OX (12 mL/kg) (Figure 1). At baseline, sickle mice exhaled 0.7-1.2 nmols/h/g CO, which increased to 4.2 nmols/h/g at 0-3 hours after MP4CO infusion (P < .025, baseline vs 0-3 hours). Exhaled CO had returned to baseline (1.1 nmols/h/g) by the time the 24- to 27-hour sample was collected. Exhaled CO was not significantly different from baseline at any time after MP4OX infusion. These data indicate that CO is rapidly bioavailable after MP4CO infusion.
Exhaled CO from NY1DD mice 24 hours prior to administration (baseline) and for 2 3-hour periods following intravenous MP4OX or MP4CO (12 mL/kg). Values are means + standard deviation (SD). N = 4 mice per treatment with the same mice used at each of the 3 time points. *P < .025 for MP4CO vs baseline.
Exhaled CO from NY1DD mice 24 hours prior to administration (baseline) and for 2 3-hour periods following intravenous MP4OX or MP4CO (12 mL/kg). Values are means + standard deviation (SD). N = 4 mice per treatment with the same mice used at each of the 3 time points. *P < .025 for MP4CO vs baseline.
MP4CO inhibits H/R-induced stasis in NY1DD sickle mice
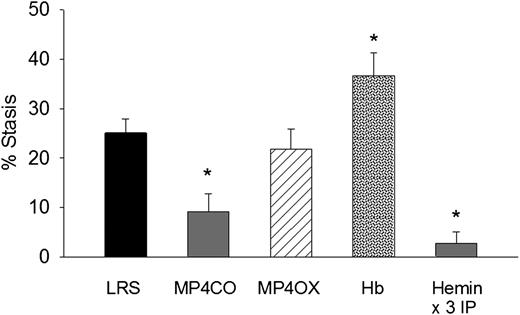
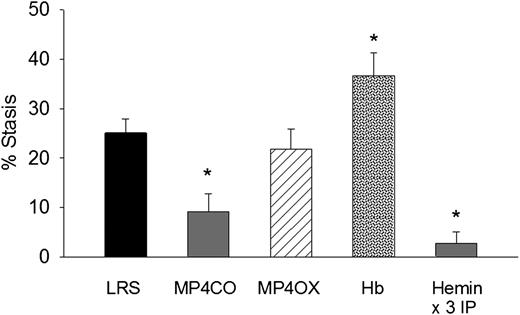
To determine the effect of MP4CO on H/R-induced stasis, we infused LRS, MP4CO, MP4OX, or Hb (8 mL/kg) into NY1DD sickle mice 24 hours before hypoxia (Figure 2). Mice infused with MP4CO had significantly less stasis (9.1%) after 1 hour of hypoxia and 1 hour reoxygenation than did mice infused with LRS (25.1%, P < .009). Stasis in mice infused with MP4OX (21.8%) was not significantly different from that of LRS-treated mice. Mice infused with Hb had significantly more stasis (36.6%) than did mice treated with LRS (P < .017). As previously shown,5 heme pretreatment IP for 3 days increases HO-1 expression several-fold in tissues and prevents H/R-induced stasis. Mice pretreated for 3 days with hemin IP had significantly lower stasis (2.8%) than did LRS-treated mice (P < .006). These data show that pretreatment with MP4CO, but not MP4OX, inhibits stasis, whereas pretreatment with Hb exacerbates stasis.
Effect of treatment pre-H/R. LRS, MP4CO, MP4OX, or Hb (8 mL/kg) was infused into NY1DD sickle mice with implanted DSFCs 24 hours before H/R. Twenty-four hours after infusion, flowing venules in the subcutaneous skin were selected and mapped. Mice were then exposed to 1 hour of hypoxia (7% O2/93% N2) and returned to room air. After 1 hour of reoxygenation, the same mapped venules were re-examined for blood flow, and the percentage of static (no flow) venules was calculated. Hemin pretreatment (40 µmols/day IP × 3 days) is a positive control known to prevent stasis by induction of HO-1. Values are means + SD. N = 4 mice per treatment except hemin (n = 6). *P < .017 vs LRS.
Effect of treatment pre-H/R. LRS, MP4CO, MP4OX, or Hb (8 mL/kg) was infused into NY1DD sickle mice with implanted DSFCs 24 hours before H/R. Twenty-four hours after infusion, flowing venules in the subcutaneous skin were selected and mapped. Mice were then exposed to 1 hour of hypoxia (7% O2/93% N2) and returned to room air. After 1 hour of reoxygenation, the same mapped venules were re-examined for blood flow, and the percentage of static (no flow) venules was calculated. Hemin pretreatment (40 µmols/day IP × 3 days) is a positive control known to prevent stasis by induction of HO-1. Values are means + SD. N = 4 mice per treatment except hemin (n = 6). *P < .017 vs LRS.
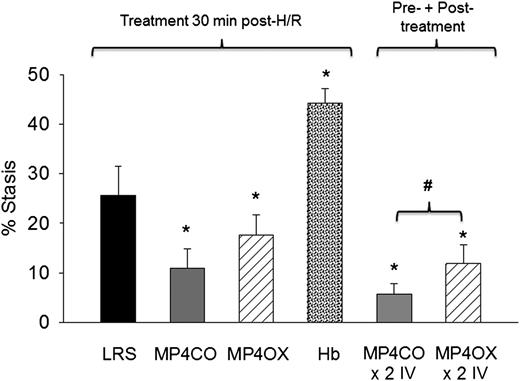
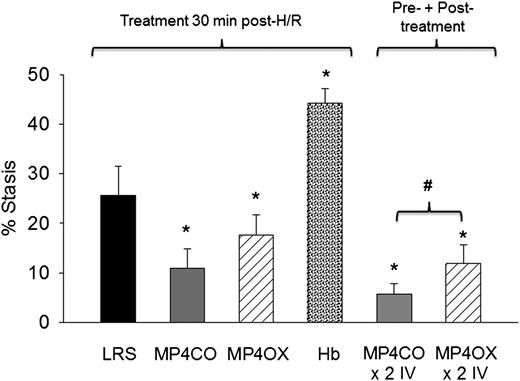
Sickle mice infused with MP4CO 30 minutes after H/R had significantly less stasis (11.0%) after 1 hour of hypoxia and 1 hour reoxygenation than did mice infused with LRS (25.6%, P < .017) (Figure 3). Stasis in sickle mice infused with MP4OX also had stasis (17.6%) lower than mice treated with LRS (P < .025), but higher than MP4CO-treated mice (P = .052). Unmodified Hb induced significantly greater stasis (44.3%) than did LRS treatment (P < .013). Sickle mice infused with MP4CO twice (×2 IV) had less stasis (5.8%) than did mice infused with MP4OX twice (×2 IV, 11.9%, P < .027) or MP4CO (P = .054) or LRS (P = .029) once.
Effect of treatment post-H/R. LRS, MP4CO, MP4OX, or Hb (8 mL/kg) was infused into NY1DD sickle mice with implanted DSFCs 30 minutes after H/R (1 hour hypoxia at 7% O2/93% N2 followed by 30 minutes in room air) during the reoxygenation phase. Microvascular stasis was measured after an additional 30 minutes in room air (1 hour reoxygenation total) as described in Figure 2. The effect of pre-H/R + post-H/R treatment is shown on the right (×2 IV) where MP4CO or MP4OX was infused 24 hours before H/R and 30 minutes after H/R. Values are means + SD. N = 4 mice per treatment. *P < .017 vs LRS; #P < .026 MP4CO vs MP4OX.
Effect of treatment post-H/R. LRS, MP4CO, MP4OX, or Hb (8 mL/kg) was infused into NY1DD sickle mice with implanted DSFCs 30 minutes after H/R (1 hour hypoxia at 7% O2/93% N2 followed by 30 minutes in room air) during the reoxygenation phase. Microvascular stasis was measured after an additional 30 minutes in room air (1 hour reoxygenation total) as described in Figure 2. The effect of pre-H/R + post-H/R treatment is shown on the right (×2 IV) where MP4CO or MP4OX was infused 24 hours before H/R and 30 minutes after H/R. Values are means + SD. N = 4 mice per treatment. *P < .017 vs LRS; #P < .026 MP4CO vs MP4OX.
MP4CO inhibits NF-ĸB activation
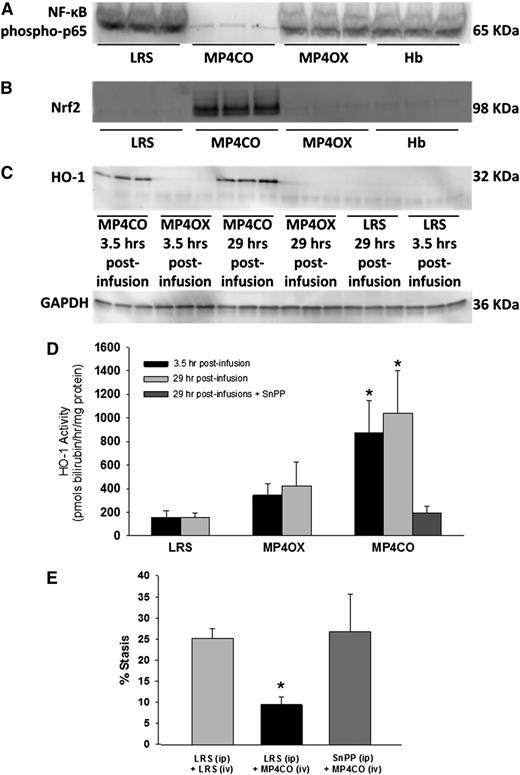
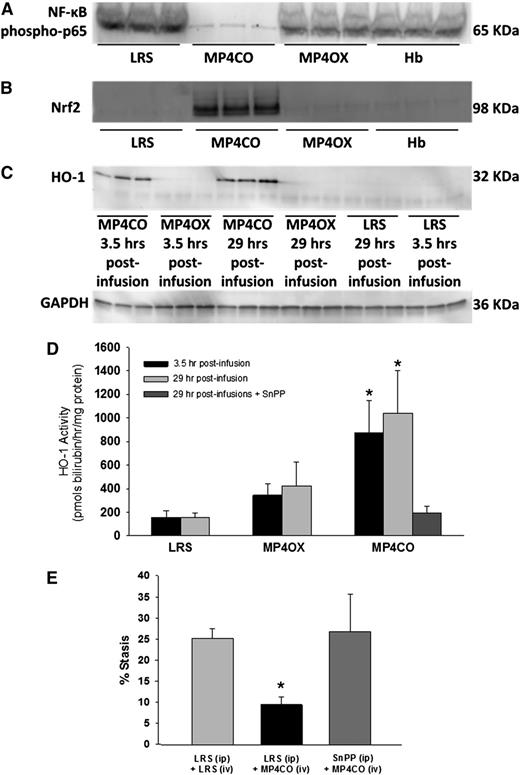
Activated nuclear NF-ĸB is a key driver of the inflammatory response. NF-ĸB expression was examined in liver nuclear extracts by western blot (Figure 4A). The livers of NY1DD mice were removed and frozen 4 hours posthypoxia, 29 hours after LRS, MP4CO, MP4OX, or Hb infusion (8 mL/kg). Activated nuclear NF-ĸB phospho-p65 was markedly reduced in the livers of sickle mice treated with MP4CO, indicating that MP4CO has a long-acting (>29 hours) inhibitory effect on NF-ĸB activation.
NY1DD sickle mice with implanted DSFCs were infused with LRS, MP4CO, MP4OX, or Hb (8 mL/kg) 24 hours prehypoxia or 30 minutes post-H/R. Livers were removed in all cases after 1 hour of hypoxia and 4 hours of reoxygenation. Thus, livers were removed 29 hours and 3.5 hours postinfusion as indicated. (A) Western blot of liver nuclear NF-ĸB phospho-p65 29 hours postinfusion. (B) Western blot of liver nuclear Nrf2 expression 29 hours postinfusion. (C) Western blot of liver microsomal HO-1 protein expression 3.5 hours and 29 hours postinfusion. (D) Liver microsomal HO activity 3.5 hours and 29 hours postinfusion and after treatment with both MP4CO (29 hours postinfusion) and pretreatment with HO inhibitor SnPP (40 µmols/kg/d × 3 days, IP). N = 4 mice/group. *P < .025. (E) Microvascular stasis after 1 hour of hypoxia and 1 hour of reoxygenation in mice treated with LRS (IP × 3 days) + LRS (IV, 8 mL/kg, 24 hours prehypoxia), LRS (IP × 3 days) + MP4CO (IV, 8 mL/kg, 24 hours prehypoxia), or SnPP (IP, 40 µmols/kg/d × 3 days) + MP4CO (IV, 8 mL/kg, 24 hours prehypoxia). N = 3 mice/group. *P < .025 LRS (IP) + MP4CO (IV) vs LRS (IP) + LRS (IV).
NY1DD sickle mice with implanted DSFCs were infused with LRS, MP4CO, MP4OX, or Hb (8 mL/kg) 24 hours prehypoxia or 30 minutes post-H/R. Livers were removed in all cases after 1 hour of hypoxia and 4 hours of reoxygenation. Thus, livers were removed 29 hours and 3.5 hours postinfusion as indicated. (A) Western blot of liver nuclear NF-ĸB phospho-p65 29 hours postinfusion. (B) Western blot of liver nuclear Nrf2 expression 29 hours postinfusion. (C) Western blot of liver microsomal HO-1 protein expression 3.5 hours and 29 hours postinfusion. (D) Liver microsomal HO activity 3.5 hours and 29 hours postinfusion and after treatment with both MP4CO (29 hours postinfusion) and pretreatment with HO inhibitor SnPP (40 µmols/kg/d × 3 days, IP). N = 4 mice/group. *P < .025. (E) Microvascular stasis after 1 hour of hypoxia and 1 hour of reoxygenation in mice treated with LRS (IP × 3 days) + LRS (IV, 8 mL/kg, 24 hours prehypoxia), LRS (IP × 3 days) + MP4CO (IV, 8 mL/kg, 24 hours prehypoxia), or SnPP (IP, 40 µmols/kg/d × 3 days) + MP4CO (IV, 8 mL/kg, 24 hours prehypoxia). N = 3 mice/group. *P < .025 LRS (IP) + MP4CO (IV) vs LRS (IP) + LRS (IV).
Thin sections of lungs and livers taken from NY1DD sickle mice treated with LRS, MP4CO, or MP4OX (8 mL/kg) 4 hours posthypoxia/29 hours posttreatment were immunostained for NF-ĸB phospho-p65 to examine NF-ĸB activation around blood vessels. In mice treated with LRS or MP4OX, NF-ĸB phospho-p65 could be seen in endothelial cells around blood vessels in the lungs (supplemental Figure 1A-B, found on the Blood website) and liver (supplemental Figure 2), as well as lung epithelial cells and hepatocytes. At higher magnification in mice treated with LRS or MP4OX, staining was primarily localized to nuclei, which indicates enhanced NF-ĸB activation in these tissues. MP4CO treatment markedly reduced NF-ĸB phospho-p65 nuclear staining in cells, indicative of decreased NF-ĸB activation in mice treated with MP4CO.
MP4CO activates Nrf2
Activated nuclear Nrf2 is an important transcriptional regulator of antioxidant response genes including HO-1. Nrf2 expression in liver nuclear extracts was examined by western blot (Figure 4B). Four hours posthypoxia—ie, 29 hours after LRS, MP4CO, MP4OX, or Hb infusion (8 mL/kg)—Nrf2 was markedly increased in MP4CO-treated sickle mice in comparison with mice treated with LRS, MP4OX, or Hb, indicating that MP4CO also has long-acting (>29 hours) effects on Nrf2 activation.
MP4CO induces HO-1
HO-1 protein expression was examined in liver microsomes by western blot (Figure 4C). Four hours posthypoxia—ie, 3.5 hours and 29 hours after LRS, MP4CO, MP4OX, or Hb infusion (8 mL/kg)—HO-1 was significantly elevated in liver microsomes after MP4CO infusion in comparison with LRS or MP4OX. Hb did not induce liver HO-1 at 24 hours (data not shown). The same liver microsomes were used to measure HO activity (Figure 4D). MP4CO treatment induced HO activity at both 3.5 hours and 29 hours postinfusion in comparison with LRS (P < .017) and MP4OX (0.025). HO activity following MP4OX infusion was not significantly different from LRS at either time.
Pretreatment of sickle mice with SnPP, a potent inhibitor of HO activity (40 µmoles/kg/d IP × 3 days), inhibited HO activity 29 hours after MP4CO infusion (8 mL/kg) in comparison with mice treated with MP4CO alone (Figure 4D; P < .025). SnPP also abrogated the protective effect of MP4CO against H/R-induced stasis (Figure 4E; P < .025).
Thin sections of lungs and livers taken from NY1DD sickle mice treated with LRS, MP4CO, and MP4OX (8 mL/kg) 4 hours posthypoxia/29 hours posttreatment were immunostained for HO-1. In mice treated with MP4CO, HO-1 expression was upregulated in all cells around blood vessels in the lungs (supplemental Figure 3A-B) and liver (supplemental Figure 4) in comparison with mice treated with LRS and MP4OX, which is consistent with reduced H/R-induced vaso-occlusion and a lower inflammatory tone in NY1DD sickle mice treated with MP4CO.
MP4CO limits cardiorespiratory collapse and mortality in HbAS mice infused with hemin
Intravenous hemin chloride has been reported to induce acute lung injury and mortality in transgenic sickle mice.18 We examined the ability of MP4CO to moderate the acute effects of intravenous hemin in heterozygous HbAS mice. These mice have a red blood cell turnover rate that is intermediate between Hb-AA and HbSS mice,19 enlarged spleens, and lower total blood Hb levels than do C57BL/6 mice (Table 1).
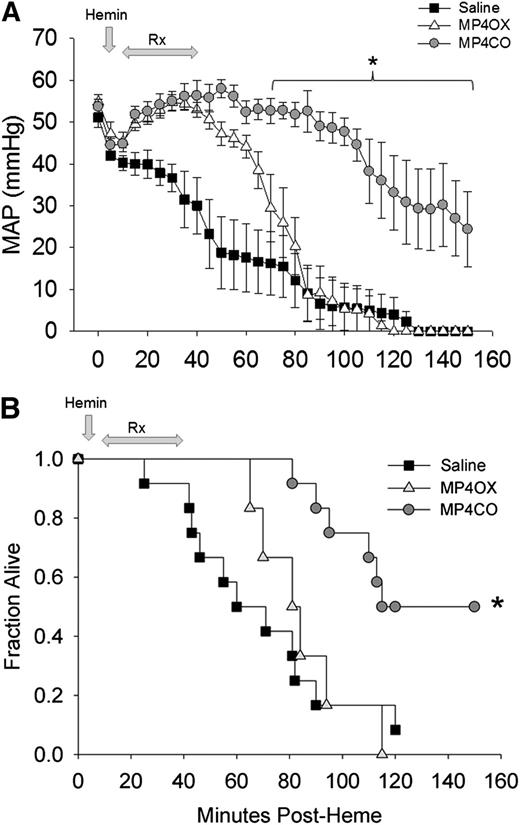
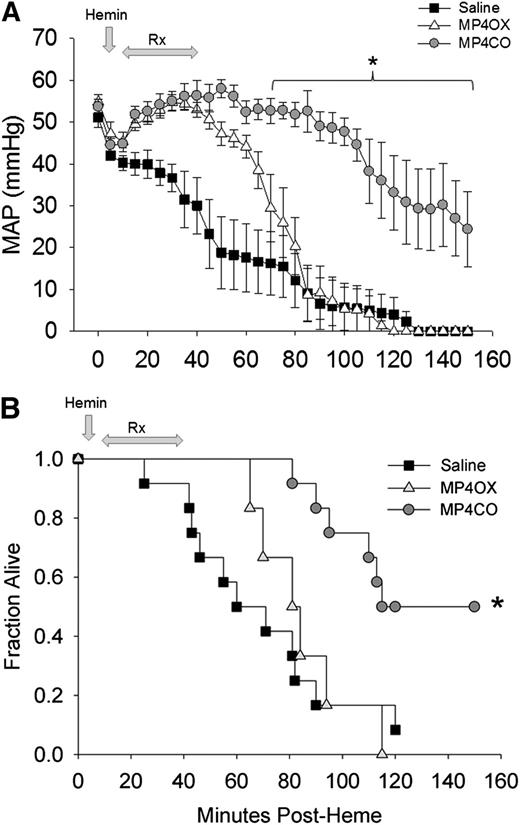
Mean arterial pressure (MAP) fell progressively from the time of hemin administration (Figure 5A). Initiation of saline infusion at 10 minutes after hemin only temporarily delayed the fall in pressure. Administration of MP4CO (8 mL/kg) improved MAP coincident with the start of MP4CO infusion, and MAP was significantly greater than that in saline-treated mice through the end of the observation period at 150 minutes. MP4CO at 4 mL/kg was less effective than at 8 mL/kg (data not shown), but still significantly greater in comparison with saline (P < .016) between 60 and 100 minutes after hemin administration. MP4OX afforded some initial protection in comparison with saline during the infusion period, but MAP in MP4OX-treated mice (8 mL/kg) quickly declined after infusion was completed. MP4OX at 2 or 4 mL/kg was not different from administration of saline (data not shown).
Effect of treatment on MAP and survival after hemin infusion into HbAS mice. (A) MAP in heterozygous HbAS mice infused with hemin followed by saline (n = 12, solid squares), MP4CO (n = 6, gray circles), or MP4OX (n = 6, open triangles). Values are means ± SD. *P < .016 vs saline. (B) Survival plot of HbAS mice infused with hemin followed by an 8 mL/kg infusion of saline (solid squares), MP4CO (gray circles), or MP4OX (open triangles). Arrows indicate the point of hemin administration and the duration of infusion of saline, MP4CO, or MP4OX. Values are fraction alive at indicated time points. *P < .016 vs saline.
Effect of treatment on MAP and survival after hemin infusion into HbAS mice. (A) MAP in heterozygous HbAS mice infused with hemin followed by saline (n = 12, solid squares), MP4CO (n = 6, gray circles), or MP4OX (n = 6, open triangles). Values are means ± SD. *P < .016 vs saline. (B) Survival plot of HbAS mice infused with hemin followed by an 8 mL/kg infusion of saline (solid squares), MP4CO (gray circles), or MP4OX (open triangles). Arrows indicate the point of hemin administration and the duration of infusion of saline, MP4CO, or MP4OX. Values are fraction alive at indicated time points. *P < .016 vs saline.
Survival following IV hemin (Figure 5B) was improved by MP4CO. One of the 12 saline-treated mice (8%) and no MP4OX-treated (0%) mice survived to the end of the 150-minute observation in comparison with 6 of 12 (50%) mice treated with 8 mL/kg of MP4CO (P < .05 vs saline, log-rank test). Administration of MP4CO at 4 mL/kg was slightly less effective on survival (38%, P < .05 vs saline) than was observed with 8 mL/kg (data not shown). Survival with MP4OX at either 4 or 8 mL/kg was not different from that of saline. MP4CO and MP4OX at 2 mL/kg were indistinguishable from saline (data not shown). Thus, MP4CO was more effective at 8 mL/kg to sustain survival and MAP, whereas MP4OX was not effective.
To examine the mechanism of MP4CO protection in HbAS mice following injection of hemin, we removed lungs and livers from treated HbAS mice at 1 hour postinfusion and immunostained for CD31, HO-1, NF-ĸB phospho-p65, P-selectin, and von Willebrand factor (VWF). HO-1 staining was present in all cells surrounding the blood vessels, including endothelial cells. HO-1 expression was highest in mice infused with hemin+MP4CO in lungs (supplemental Figure 5A-B) and liver (supplemental Figure 6) in comparison with all other treatments including hemin + saline. HO-1 staining was lowest in mice treated with saline or hemin + MP4OX, with little to no HO-1 staining around the blood vessels. Lungs and livers from mice treated with hemin + saline had intermediate levels of HO-1 expression.
NF-ĸB phospho-p65 staining was highest in the lungs (supplemental Figure 7A-B) and livers (supplemental Figure 8) of mice treated with hemin + saline. At higher magnification, staining is apparent in the nuclei and cytoplasm of cells surrounding pulmonary vessels including endothelial cells. In the liver, NF-ĸB phospho-p65 was primarily nuclear with some cytoplasmic staining. There was a marked decrease in nuclear and cytoplasmic NF-ĸB phospho-p65 staining with hemin + MP4CO.
Hemin infusion induces profound vaso-occlusion in HbSS and HbAS mice that requires cell surface expression of endothelial cell Weibel Palade body constituents P-selectin and VWF.20 P-selectin and VWF expression on the cell surface of vessels in the lungs (supplemental Figure 9A,B) and livers (supplemental Figure 10) was stimulated in HbAS mice infused with hemin + saline and hemin + MP4OX and was markedly decreased in mice infused with saline or hemin + MP4CO. Thus, protection from hemin lethality by MP4CO correlates with a rapid (≤1 hour) increase in HO-1, a reduction in NF-ĸB activation, and decreased P-selectin and VWF on blood vessels in the lungs and liver.
Discussion
The effects of CO in alleviating the consequences of SCD have been studied for a number of years.5,6,21 CO inhibits Hb-S polymerization, red cell shape change, and vaso-occlusion by direct actions on HbS and also via indirect actions preventing red cell lysis, inflammation, adhesion, and apoptosis, and by activating guanylate cyclase leading to vasodilation. These actions of CO are also elicited by increased expression and activity of HO-1, leading to the conclusion that production of CO and biliverdin and induced-ferritin iron sequestration are beneficial in a number of hemolytic scenarios.4,22 The current results corroborate previous data from inhaled CO and demonstrate (1) the unique observation that intravenous administration of exogenous CO protects sickle mice from the vascular effects of the pro-oxidant stimuli hypoxia or hemin; (2) that the increased expression and activity of HO-1 are integral in the mechanism of protective effects against hypoxia; and (3) that the mechanism of this effect may be through CO-mediated increases in nuclear Nrf2, enhanced expression and activity of HO-1, and decreases in NF-ĸB activation and expression of adhesion molecules such as P-selectin and VWF on the vessel wall.
Delivery of CO in animals is accomplished typically using the inhaled gas,23 although pharmacologic effects have been demonstrated with CO-releasing molecules,24 cell-free CO-Hb,11,25 and CO-saturated red blood cells.26 MP4CO is a solution of human Hb tetrameter conjugated with 5 kD PEG on the 4 globin chains and saturated with 100% CO.11 MP4OX is the identical molecule saturated with oxygen.12 The CO from MP4CO rapidly distributes in blood and has a half-life in circulation of approximately 90 minutes26 (Cabrales et al and unpublished data). After the release and distribution of CO from MP4CO, the fate of the PEG-Hb is identical to that of MP4OX, ie, uptake by tissue macrophages with predominantly renal excretion over the ensuing days. The half-life of the PEG-Hb moiety is approximately 20 hours in rats and humans.27 Comparison of MP4CO and MP4OX in both the NY1DD and the HbAS mice allows clear distinction of CO gas from the solution properties of PEG-Hb, which includes effects on plasma volume and peripheral perfusion demonstrated previously with MP4OX.28 The bioavailability of CO from MP4CO is demonstrated in the current study by the elevated concentration of CO in expired air from sickle mice treated with MP4CO, but not with MP4OX. Although the tissue distribution of CO is unknown, our data demonstrate that approximately 50% of CO from MP4CO is exhaled within 3 hours of administration, suggesting that CO freely dissociates from MP4CO and is available to bind native red cell Hb or distribute into endothelial cells and other tissues. We have demonstrated that MP4CO reduced myocardial infarct size after ischemia/reperfusion in rats, confirming the cytoprotective effects of CO demonstrated by others.25,29 In the present study, MP4CO inhibited venular stasis in NY1DD mice in response to H/R and reduced cardiovascular collapse and mortality in HbAS mice in response to hemin, neither of which was observed following administration of MP4OX. Similarly, MP4CO substantially reduced NF-ĸB activation, increased HO-1 expression and activity, and increased nuclear Nrf2, none of which was observed with MP4OX. Thus the observed effects are a result of CO released from MP4CO and not from the heme load (or ancillary hemodynamic and volume effects) introduced by MP4OX, MP4CO, or Hb. MP4CO infusion elicited a peak CO-Hb concentration of 6.5% following a 12 mL/kg dose, consistent with the demonstration of CO-mediated cytoprotection with CO-Hb saturation near 5%.29,30 It is also important to note that the oxygenated molecule, MP4OX, showed a slight tendency to reduce venular stasis, and unmodified Hb exacerbated stasis when administered pre- or post-H/R. MP4CO and MP4OX appear less of an oxidant stimulus than does unmodified Hb, perhaps because of differences in size, extravasation, heme retention, solution properties, or the high nitrite reductase activity imparted by the stabilized R-state conformation and low P50 of the PEG-Hb molecule.31
A number of mechanisms may contribute to the observed effects of MP4CO. CO potentially prevents HbS polymerization and increases the delay time for red cell shape change.32 The CO administered in the current study is approximately 1/20 of the molar heme present in the red blood cells of mice. Dasgupta et al33 reported a 30% attenuation of red cell sickling by increased expression of Hb-F in sickle mice to 21%. Similarly, hydroxyurea is thought to reduce sickle crisis events only when Hb-F is elevated to 15% to 20% of total Hb.34 Thus, it is difficult to envision that a maximum of 6.5% CO-Hb saturation with MP4CO favors inhibition of HbS polymerization in the current studies. Moreover, the current observations of reduced vascular stasis 24 hours after dosing MP4CO are unlikely because of direct CO binding to HbS, because the short residence time of CO leaves little CO-Hb in blood beyond 90 minutes after dosing.
Our work has focused on an alternative mechanism for the actions of CO, based on the chronic hemolysis and enhanced oxidative and inflammatory state in SCD.35 Elevated plasma Hb generates reactive oxygen and nitrogen species from nitric oxide binding, oxidation of the globin moiety, generation of free heme, lipid peroxidation, and iron-catalyzed oxidation,36,37 promoting vascular consequences in transgenic sickle cell mice characterized by cellular adhesion/aggregation, vaso-occlusion, and increased mortality. Counteracting these deleterious actions, an important protective consequence of hemolysis, is the induction of HO-1 to limit the toxicity of free heme by a number of mechanisms, including the induction of ferritin and release of protective products biliverdin and CO.35 Although basal HO-1 expression is elevated in transgenic sickle mice,4,5 additional protective effects are observed by HO-1 gene therapy17,38 or IP administration of low-dose exogenous heme,5 such that therapeutic effects might be expected from increased activity of HO-1. The current results with IV MP4CO corroborate observations that inhaled CO protects transgenic sickle mice from adverse effects of oxidant stimuli4,5 and agree with numerous studies documenting the beneficial effects of HO-1 overexpression or administration of the enzymatic products, CO and biliverdin.24
Candidate mechanisms for the beneficial effects of CO include binding to cell-free deoxyHb to stabilize and prevent heme loss,9 upregulation of antioxidant and anti-inflammatory pathways,39,40 and inhibition of NF-ĸB-mediated inflammation.5,41 We have previously demonstrated that inhaled CO and overexpression of HO-1 prevent vascular stasis and are associated with activated p38 MAP kinase and Akt pathways, reduction in activated NF-ĸB, and decreased circulating adhesion molecules.5,17 The current results are consistent with a lower overall inflammatory state in mice receiving MP4CO as evidenced by increased HO-1 expression, decreased nuclear NF-ĸB phospho-p65, and the inhibition of cell-surface P-selectin and VWF on endothelium throughout the lungs and liver. These observed anti-inflammatory effects by MP4CO are relevant to the decrease in vaso-occlusion and protection from hemin lethality and the fall in MAP. This is supported by the concurrent increase of nuclear Nrf2 with administration of MP4CO. CO plays a central role in nuclear Nrf2 localization and induction of HO-110 and other antioxidant enzymes.39 CO-mediated Nrf2 translocation confers cytoprotection42 and anti-inflammatory actions.39,40 The importance of Nrf2 in HO-1 expression is highlighted by the low expression of HO-1 and poor survival of Nrf2 knockout mice following infection with Plasmodium berghei4 or Escherichia coli.40 Our observation that the actions of MP4CO were prevented by SnPP suggests that HO activity is critical for prevention of stasis by CO and that CO acts intracellularly, primarily by a feed-forward mechanism to amplify the expression and protective actions of HO-1, including further generation of CO. This concept is supported by earlier observations that HO-1 expression is an intermediate step in the anti-inflammatory activity of CO40,43 and that endogenous, physiologic concentrations of CO are sufficient to elicit cellular signaling events.42,44
The varied responses of sickle mice to the different Hb molecules in this study are noteworthy. MP4CO decreased NF-ĸB activation, increased HO-1 expression, inhibited P-selectin and VWF expression on endothelial cells, and reduced vascular stasis, whereas MP4OX had little effect on NF-ĸB, HO-1, and P-selectin/VWF and a smaller effect on stasis. Unmodified Hb had no effect on HO-1 in liver and dramatically enhanced stasis following H/R. These differences existed despite equal doses of Hb administered with each test solution. The enhanced stasis with unmodified Hb is likely due to decreased stability in vivo, with more rapid dimerization, greater heme loss,45,46 and a greater resultant activation of the vascular endothelium.20,47 The differential effect of these solutions on HO-1 induction could be explained by differences in the time course and magnitude of CO or heme concentration available to induce expression. For example, the effects of Hb, but not CO, might be expected to be modulated by haptoglobin or hemopexin.48 Because both unmodified Hb and pegylated Hb complex with haptoglobin and interact with the CD163 receptor (D.J. Schaer, Division of Internal Medicine, University Hospital, Zurich, Switzerland, personal communication, April 13, 2013), the general disposition of Hb is unlikely to be different for the 3 solutions. Alternatively, differences may exist in the potency and mechanism of HO-1 induction for CO, Hb, and heme via Nrf2 and Bach-1 regulation.49,50 The differential effects of CO versus Hb or heme on HO-1 induction will require further study.
The events precipitating a vaso-occlusive crisis in patients are not well delineated. Our results demonstrate that MP4CO inhibits the vascular consequences of 2 different stimuli, ie, hypoxia and hemin, generated in 2 different transgenic sickle models. Intravenous hemin is known to induce acute lung injury, hypotension, severe vaso-occlusion, and death in sickle mice.18,20 Heme toxicity may be ameliorated by rapid (≤1 hour) HO-1 induction by CO, which would enhance heme degradation, generate more CO, inhibit vaso-occlusion, and blunt NF-ĸB-driven inflammatory responses.6,20 An integral role of reduced NF-ĸB activation in the effects of CO/Nrf2/HO-1 is supported by the current results and those of others.38,51 CO41 and Nrf252 may play important roles in modulating toll-like receptor 4 (TLR4) signaling. Recently, Belcher and colleagues20 proposed a pathway by which hemolysis, and oxidative/inflammatory heme, triggers vaso-occlusion via endothelial TLR4 signaling, NF-ĸB activation, PKC signaling, and exocytosis of Weibel Palade body P-selectin and VWF. Thus, while still speculative, a potential pathway is emerging that links our current observations to inhibition of the TLR4 pathway. TLR4 signaling can be initiated by lipopolysaccharide, heme, or H/R.20,53 CO broadly suppresses the inflammatory response of human monocytes to lipopolysaccharide by reshaping events in TLR4 signal transduction such as stress kinase responses and NF-ĸB activation.41 CO suppression of TLR4-mediated signaling events, especially in the pulmonary vasculature, may explain the reduced mortality and blunted fall in MAP in the HbAS mice in response to heme. The current data demonstrate that CO is effective in episodes of inflammation initiated by either H/R or hemin administration and suggest that the durable expression of Nrf2 and HO-1 observed in response to CO might play a tonic suppression role in limiting the evolution of acute vaso-occlusive crisis.
In summary, the current results present evidence that MP4CO inhibits the effects of stimuli that induce vaso-occlusive crises in transgenic sickle mice. The actions of MP4CO are attributed to CO, because the identical molecule delivering oxygen did not elicit the same results. Our results document the upregulation of Nrf2 and HO-1 with administration of CO and support the extensive body of work implicating CO, Nrf2, and HO-1 as potential therapeutic targets. Recent clinical trials of agents intended to alleviate or shorten the duration of sickle cell crisis have failed to demonstrate robust and clinically meaningful effects.54,55 The current results extend work begun with CO in sickle disease more than a half-century ago21 and offer MP4CO as a potential therapy to limit the evolution and duration of painful vaso-occlusive events in patients with SCD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is an Inside Blood commentary on this article in this issue.
Acknowledgments
The authors thank Dr Robert Hebbel and Fuad Abdulla for breeding and phenotyping the NY1DD mice, Stephanie Spann and Rachell Sigan for phenotyping the HbAS mice, and Dr Tim Townes and the University of Alabama, Birmingham Research Foundation, for license to the HbAS mice. We also thank Dr Michael Levitt and Julie Furne for measurement of expired CO and Phong Nguyen and Minh Nguyen for preparation of the immunohistochemistry panels in the online supplemental Data.
This research was funded by a grant from Sangart, Inc., to G.M.V. and J.D.B.
Authorship
Contribution: J.D.B., M.Y., K.B., and G.M.V. designed experiments, analyzed data, and wrote the paper; C.C., J.N., and P.T. collected the data; and M.Y. and K.B. also provided valuable reagents (MP4CO, MP4OX, and Hb).
Conflict-of interest-disclosure: M.Y., K.B., and P.T. are employees of Sangart, Inc. The remaining authors declare no competing financial interests.
Correspondence: John D. Belcher, University of Minnesota School of Medicine, 420 Delaware St SE, MMC 480, Minneapolis, MN 55455; e-mail: belcher@umn.edu.