Key Points
Anaplastic large-cell lymphoma has a unique miRNA signature.
The miR-17∼92 is an important downstream effector of ALK oncogenic pathway.
Abstract
Anaplastic large-cell lymphomas (ALCLs) encompass at least 2 systemic diseases distinguished by the presence or absence of anaplastic lymphoma kinase (ALK) expression. We performed genome-wide microRNA (miRNA) profiling on 33 ALK-positive (ALK[+]) ALCLs, 25 ALK-negative (ALK[−]) ALCLs, 9 angioimmunoblastic T-cell lymphomas, 11 peripheral T-cell lymphomas not otherwise specified (PTCLNOS), and normal T cells, and demonstrated that ALCLs express many of the miRNAs that are highly expressed in normal T cells with the prominent exception of miR-146a. Unsupervised hierarchical clustering demonstrated distinct clustering of ALCL, PTCL-NOS, and the AITL subtype of PTCL. Cases of ALK(+) ALCL and ALK(–) ALCL were interspersed in unsupervised analysis, suggesting a close relationship at the molecular level. We identified an miRNA signature of 7 miRNAs (5 upregulated: miR-512-3p, miR-886-5p, miR-886-3p, miR-708, miR-135b; 2 downregulated: miR-146a, miR-155) significantly associated with ALK(+) ALCL cases. In addition, we derived an 11-miRNA signature (4 upregulated: miR-210, miR-197, miR-191, miR-512-3p; 7 downregulated: miR-451, miR-146a, miR-22, miR-455-3p, miR-455-5p, miR-143, miR-494) that differentiates ALK(–) ALCL from other PTCLs. Our in vitro studies identified a set of 32 miRNAs associated with ALK expression. Of these, the miR-17∼92 cluster and its paralogues were also highly expressed in ALK(+) ALCL and may represent important downstream effectors of the ALK oncogenic pathway.
Introduction
Anaplastic large-cell lymphomas (ALCLs) are aggressive T-cell neoplasms typically composed of cohesive clusters of large cells with abundant cytoplasm and eccentric horseshoe or kidney shaped nuclei.1 The tumor cells show strong, uniform expression of CD30, 1 or more T-cell antigens, epithelial membrane antigen (EMA or MUC1), and cytotoxic cell-associated antigens (eg, TIA-1, granzyme B, and/or perforin).1 The 2 major subgroups of ALCL recognized by the World Health Organization (WHO) show similar morphologic and immunophenotypic features and are classified based on the presence or absence of chromosomal translocations involving the anaplastic lymphoma kinase (ALK) gene located at the chromosome 2p23 locus.1 The translocations result in aberrant ALK expression with NPM1 as the major fusion partner due to t(2;5)(p23;q35) in ALK-positive (ALK[+]) ALCL.2 This genetic alteration leads to STAT3 activation via phosphorylation by the NPM-ALK chimeric protein, which is critical for the maintenance of the neoplastic phenotype.3 A majority of the patients with ALK(+) ALCL are young and have a significantly better clinical outcome than patients with systemic ALK-negative (ALK[–]) ALCL.4,5
The systemic ALK(–) ALCL1,2,6 subgroup lacks ALK expression and has been included as a provisional pathologic entity in the current WHO classification.1 The lack of a defining biomarker or genetic abnormality in ALK(–) ALCL makes the diagnosis challenging.5,7 Some investigators argue that ALK(–) ALCLs may represent a morphologic variant within the heterogeneous category of peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) based on a lack of clear biological differences between them.8,9 Although several gene expression profiling and comparative genomic hybridization studies have shown that ALK(+) ALCL and ALK(–) ALCL have distinct patterns of expression signatures and genomic aberrations,10-14 there are many overlapping molecular features,10-15 including shared expression of a number of genes.16,17 Recently, recurrent translocations t(6;14)(p25;q11.2) involving IRF418 and t(6;7)(p25.3;q32.3) involving DUSP2219 in ALK(–) ALCL have been identified, supporting that ALK(–) ALCL is a distinct entity.5,11,17 However, these abnormalities are restricted to only a small subset (4% to 10%) of the ALK(–) ALCL cases.18,19
MicroRNAs (miRNAs) are emerging as tissue-specific biomarkers with the potential for clinical application in identifying cancer subtypes.20 More recently, several miRNAs such as miR-101,21 miR-16,22 miR-135b,23 and miR-29a24 have been demonstrated to have a role in the pathogenesis of ALK(+) ALCL. However diagnostic signatures for the 2 subgroups of ALCL have not been defined. We have performed a large-scale global analysis of miRNA expression in ALCL, including 58 ALCLs, 20 PTCLs, normal CD3+ T cells, and stromal cells, by using a platform based on high-throughput TaqMan quantitative real-time PCR (qRT-PCR). Here, we report the miRNA signatures associated with the 2 systemic forms of ALCL and functional attributes of miRNA expression in ALCL.
Materials and methods
Patient specimens, cell lines, and normal cells
Tumor specimens from patients with ALK(+) ALCL (n = 33), ALK(–) ALCL (n = 25), angioimmunoblastic T-cell lymphoma (AITL; n = 9), and PTCL-NOS (n = 11) were obtained from 4 institutions (University of Torino, Torino, Italy; Memorial Sloan-Kettering Cancer Center, New York, NY; Children’s Oncology Group, Monrovia, CA; and University of Nebraska Medical Center, Omaha, NE) with informed patient consent in accordance with the Declaration of Helsinki and approval by the local institutional review boards. The cases were diagnosed by expert hematopathologists in accordance with the WHO classification.1 The clinical and molecular data, including ALK-translocation status and immunohistochemical (IHC) profiles are listed in Table 1. The miRNA profiles of B-cell lymphomas were used for comparative analysis and have been previously described.25
The NPM-ALK(+) ALCL cell lines (SUP-M2/TS, Karpas 299, L82, JB6, and SUDHL-1) and ALK(–) ALCL cell line (MAC-1) were cultured in RPMI-1640 medium containing 10% fetal calf serum (Lonza, Rockland, ME), 2 mM l-glutamine, and 1% streptomycin. Normal T-cell CD3+ and B-cell subsets25 were obtained from fresh tonsils as described previously26 by using magnetic MACS microbeads according to the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA), and pooled RNA and miRNA from at least 6 to 9 healthy individuals was profiled in 3 different experimental settings. The stromal cells were isolated from minced human tonsils digested with 2 mg/mL collagenase type IV (Worthington Biochemical, Freehold, NJ) and 0.1 mg/mL DNase I (Sigma-Aldrich, St. Louis, MO) and processed as described previously.27
Knockdown of ALK in ALCL cell lines
Short hairpin (shRNA) directed against the cytoplasmic domain of ALK was used to knock down (KD) ALK in SUP-M2/TS cells, as done previously.17,28 The expression plasmid for inducible NPM-ALK silencing was produced by subcloning the H1 promoter-ALK-shRNA cassette into the pLVTH vector as described previously.28 shRNA expression was induced by the presence of doxycycline (1 μg/mL). The KD efficiency of the shRNA was estimated by the qRT-PCR method as described by Agnelli et al.16 Briefly, 100 ng of total RNA extracted from transduced cells was reverse transcribed with the miScript Reverse Transcription Kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions. qRT-PCR assays were performed in triplicate in the Thermal iCycler (Bio-Rad) and were normalized to GAPDH, and relative expression of ALK was calculated by using the standard ΔCT method as described by Agnelli et al.16 The miRNA differential expression between control cell lines and KD cell lines was evaluated by using standard statistical tests as described in the “miRNA profiling data analysis” section.
Conditional KD of miR-17∼92 in ALK(+) ALCL cell lines
For KD of miR-17∼92, Karpas 299 and JB6 were transduced with lentivirus packaged with the pTRIPZ sponge construct, pMD2G envelope vector, and psPAX2 packaging plasmid in HEK293 T cells as described previously.29 Transduced cells were selected with puromycin, and doxycycline-induced green fluorescent protein (GFP)-expressing cells were isolated by flow cytometry (FACSCalibur; BD Biosciences). The cell cycle analysis of transduced cells was conducted by using Hoechst 33342 staining (H3570; Invitrogen, Carlsbad, CA). Apoptosis of cells was determined by staining with Annexin V-PE (Apoptosis Detection Kit; BD Pharmingen) according to the manufacturer’s instructions. Both cell cycle and apoptosis assays of transduced cells (ie, GFP+ cells) were analyzed by flow cytometry (FACSCalibur) as described previously.29
Treatment of ALK(+) ALCL cell lines with STAT3 inhibitor
The viability of cell lines (Karpas 299, JB6, L82) after treatment with the STAT3 small-molecule inhibitor Stattic (Calbiochem, Billerica, MA), which blocks STAT3 phosphorylation at Tyr705, was determined by using the CellTiter 96 Aqueous kit (Promega, Madison, WI) according to the manufacturer’s instructions29 in a time- (12 to 24 hours) and dose- (1 to 5 μM) dependent manner. The antibodies for immunoblotting in this study were anti-PTEN, anti-STAT3 (total), anti-STAT3 (phosphorylated), anti–p-AKTser473 (Cell Signaling Technology, Beverly, MA), and anti–β-actin (Santa Cruz Biotechnology, Paso Robles, CA). The qRT-PCR of miR-17∼92 cluster members (miR-19a and miR-92) was performed as described by Agnelli et al.16
RNA isolation and miRNA profiling
Total RNA for miRNA profiling was extracted from formalin-fixed paraffin-embedded (FFPE) tissues using 2 tissue cores (∼1 mm2 diameter) from an area with predominant tumor cells (>70%), by using the RecoverAll total nucleic acid isolation kit. RNA was obtained from cell lines and normal cells with an mirVana miRNA isolation kit according to the manufacturer’s instructions (Ambion, Austin, TX). Reverse transcription was carried out by using input amounts of 300 ng of total RNA from cell lines and normal cells and 100 ng of total RNA from FFPE samples using the Megaplex RT Primers and enzyme kit. This was followed by a subsequent step of preamplification (12 cycles) using Megaplex PreAmp Primers to enhance assay sensitivity, as recommended by the manufacturer (ABI, Foster City, CA). The preamplified complementary DNAs were loaded onto 384-well format miRNA assays plates (TaqMan Array Human MicoRNA A Card, V2.0; ABI), and then qRT-PCR was performed on a 7900HT Fast Real-Time PCR System (ABI). The threshold cycle was defined as the fractional cycle number at which the fluorescence exceeds the fixed threshold of 0.1 with an automatic baseline using the RQ Manager version 1.2 software according to the manufacturer’s instructions (ABI).
IHC staining, fluorescence in situ hybridization, TCR-γ gene rearrangement analysis
IHC stains for T-cell markers, including CD3, CD2, CD4, CD5, CD8, and CD43, cytotoxic markers (TIA-1, granzyme B, and perforin), and CD30 were performed on FFPE tissue sections as described previously.30 For IHC staining of ALK, the rabbit monoclonal antibody SP8 against ALK1 was used on a Ventana ES automated immunostainer (Ventana Biotek, Tucson, AZ) with a streptavidin-biotin peroxidase detection system. Positive signals were localized in nucleus, nucleolus, cytoplasm, and/or membrane, with different staining patterns indicating different translocation variants. ALK translocation was detected at initial cytogenetics review or by using a commercially available LSI ALK Dual Color Break Apart Probe (Vysis, Downers Grove, IL) according to the manufacturer’s instructions. Analysis of T-cell receptor γ (TCR-γ) gene rearrangement using PCR-based methods was performed on a subset of cases with adequate materials as reported previously.31
miRNA profiling data analysis
The raw data were uploaded into BRB-ArrayTools version 3.9.032 for analysis. Briefly, we performed global median normalization for the entire data set from FFPE cases prior to any further analysis. To select miRNAs for analysis, we used 3 approaches: (1) exclude miRNA showing minimal variation across the arrays from analysis by including only miRNAs whose expression differed by at least twofold from the median in at least 10% of the cases, (2) exclude miRNAs if the log intensity variation was not significant (P > .05) compared with the median of all the variances, and (3) CT = 30 or higher was used as the threshold for the minimum level of expression. For the CD3+ T-cell–specific miRNA signature, we used the average difference (>2 CT value difference) and Student t test (P < .01) between CT values of CD3+ T cells and other normal cells examined, and fold difference was expressed by converting the log scale (ΔCT) to a normal scale. The miRNA classifier for ALK(+) ALCLs and ALK(–) ALCLs was constructed by using a Bayesian algorithm that estimated the probability of a case belonging to 1 subtype compared with another subtype as described earlier.33 In our series, differentially expressed miRNAs were selected at a significance of P < .05 and a mean fold-difference >4 between any 2 group comparisons. The miRNA data from fresh-frozen cell lines or normal cells was used for comparative analysis only.
Survival analysis
Event-free survival (event was defined as progression or death from any cause after the start of chemotherapy) and overall survival (event was defined as death from any cause) were estimated by using the Kaplan-Meier method, and differences were assessed by using the log-rank test.
Results
Patient characteristics
The clinical and pathological characteristics of patients included in the study are summarized in Table 1. As expected, the majority of the ALK(+) ALCL patients were younger, with a median age of 18 years (range, 3 to 62 years), with a marginal male predominance (M:F ratio, 1.31) and significantly better clinical outcome (P < .05) compared with ALK(–) ALCL and other PTCL patients (supplemental Figure 1). The ALK(–) ALCL patients were older, with a median age of 60 years (range, 16 to 84 years) at time of diagnosis and prominent male predominance (M:F ratio, 4.75). The IHC profile showed characteristic antigen profiles in ALK(+) ALCL and ALK(–) ALCL patients with abnormal expression of pan T-cell markers (Table 1). The expression of at least 1 cytotoxic marker (ie, TIA-1, granzyme B, or perforin) was more frequently observed in ALK(+) large-cell lymphomas (78%; 14 of 18) than ALK(–) ALCL patients (52.63%; 10 of 19). There was strong and uniform expression of CD30 in all ALCL patients. The t(2;5) was observed by fluorescence in-situ hybridization in 91.67% (11 of 12) of ALK(+) ALCL patients, while the remaining patient was evaluated by IHC staining alone and was positive for ALK expression. Clonal TCR gene rearrangements were observed in the majority of evaluable patients (Table 1). The immunophenotypic profiles of other PTCL patients were consistent with their diagnosis and were distinct from the ALCL patients (Table 1).
Identification of normal CD3+ T-cell–specific miRNA signature and its expression in ALCL and PTCL patients
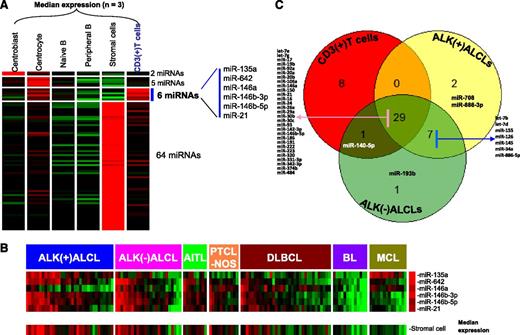
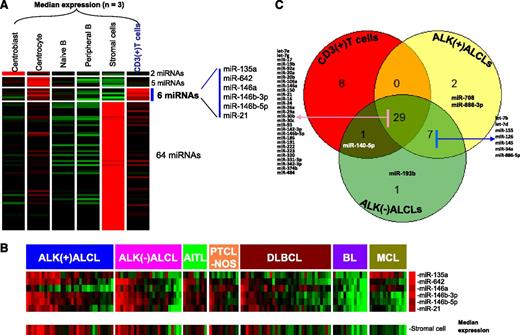
We identified a pan T-cell miRNA signature by comparing the miRNA profiles of normal CD3+ T cells with normal B-cell subsets (centroblast, centrocyte, naive B cell, and peripheral B cell) and stromal cells. We identified 6 miRNAs that showed significantly higher expression (P < .01 and greater than fourfold change) in CD3+ T cells (Figure 1A). Although 5 of 6 miRNAs (miR-135a, miR-146a, miR-146b-3p, miR-146b-5p, and miR-21) have been associated with T cells in previous studies,34,35 we identified 1 novel miRNA (miR-642) expressed in T cells that has not been previously reported. When the expression of these miRNAs was correlated with ALCL and other PTCLs and further compared with B-cell lymphoma entities (diffuse large B-cell lymphoma [DLBCL], Burkitt lymphoma, and mantle cell lymphoma), we observed that this miRNA signature was enriched in ALCL, PTCLs, and some cases of DLBCL that correlated with the T-cell and stromal cell content of the tumor, probably representing T-cell–rich DLBCLs (Figure 1B). Although expression of many T-cell antigens is lost in ALCL,36 our T-cell miRNA signature was consistently expressed in the majority of the ALCLs at a level comparable to that in other PTCLs. However, the consistent low expression of miR-146a in the majority of ALK(+) ALCLs and in a subset of ALK(–) ALCLs was of special interest. In contrast, miR-135a expression was more often low in the other PTCLs and unexpectedly high in mantle cell lymphoma.
Identification of T-cell–associated miRNA signature. (A) The 6-miRNA signature differentially expressed (P < .01; greater than fourfold change) in normal T cells compared with B-cell subsets and stromal cells. (B) Expression of T-cell miRNA signature in different subtypes of T-cell and B-cell lymphomas. A majority of PTCL cases (including ALK(+) ALCL, ALK(–) ALCL, AITL, and PTCL-NOS) and a few DLBCL cases showed enrichment of T-cell miRNA as well as stromal-cell miRNA signatures. (C) Overlap in the expression of the top 10% of miRNAs from CD3+ T cells, ALK(+) ALCLs, and ALK(–) ALCLs. BL, Burkitt lymphoma; MCL, mantle cell lymphoma.
Identification of T-cell–associated miRNA signature. (A) The 6-miRNA signature differentially expressed (P < .01; greater than fourfold change) in normal T cells compared with B-cell subsets and stromal cells. (B) Expression of T-cell miRNA signature in different subtypes of T-cell and B-cell lymphomas. A majority of PTCL cases (including ALK(+) ALCL, ALK(–) ALCL, AITL, and PTCL-NOS) and a few DLBCL cases showed enrichment of T-cell miRNA as well as stromal-cell miRNA signatures. (C) Overlap in the expression of the top 10% of miRNAs from CD3+ T cells, ALK(+) ALCLs, and ALK(–) ALCLs. BL, Burkitt lymphoma; MCL, mantle cell lymphoma.
In further analysis, we ranked miRNAs in the order of their expression level in normal T cells. Of the top 10% of highly expressed miRNAs (38), a substantial proportion (76.3%; 29 of 38) was also represented in the top 10% of highly expressed miRNAs in both ALCL subgroups. This suggests that ALCL maintains a substantial T-cell–related miRNA profile (Figure 1C). Seven other miRNAs (miR-7b, miR-7d, miR-155, miR-126, miR-145, miR-34a, and miR-886-5p) were shared by both ALCL subgroups. When we examined the enriched miRNAs in normal T cells (upper half by expression level), we observed 7 miRNAs (supplemental Table 1) that were significantly expressed at lower levels (P < .05 and greater than fourfold) in ALCL, indicating loss of function of these miRNAs. In contrast, 18 miRNAs enriched in ALCL entities were not expressed in normal T cells or stromal cells, with miR-10b and miR-512-3p showing >100-fold change (supplemental Table 1), suggesting an abnormal gain of function of these miRNAs. A small subset of 7 miRNAs were specifically enriched in only ALK(+) ALCLs, and 3 miRNAs were specifically enriched in ALK(–) ALCL compared with normal T cells (supplemental Table 1).
In vitro analysis of ALK-associated miRNA signature
We determined the ALK-associated miRNA signature by KD of ALK by specific shRNAs in an ALK(+) ALCL cell line (SUP-M2/TS cells) resulting in at least a 90% decrease in ALK mRNA as shown in supplemental Figure 2. The differentially expressed miRNAs (supplemental Table 2) after ALK KD compared with control included 22 downregulated miRNAs mainly associated with cell proliferation such as let-7d, miR-01, and miR-17∼92 cluster and its paralogues (miR-93, miR-363, miR-106a) consistent with the role of ALK in inducing cell proliferation. We correlated this miRNA signature from the KD experiments with the miRNA profiles of ALK(+) ALCLs vs ALK(–) ALCLs and PTCL entities, and only a fraction of the miRNAs demonstrated the expected changes in ALCL patient samples. These include miR-629, miR-886, miR-93, members of the miR17∼92 cluster, and miR-106 on ALK KD (supplemental Table 2).
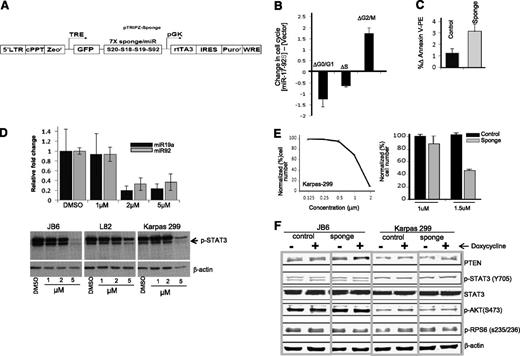
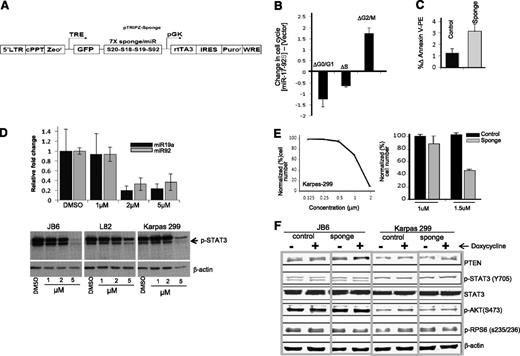
To further validate the functional effects of miR-17∼92 in vitro, we analyzed the functional changes of reducing miR-17∼92 in Karpas 299 by a sponge construct that sequesters the corresponding miRNAs29 (Figure 2A). Lentiviral transduction of an miR-17∼92 sponge resulted in an increased percentage of cells in G2→M phase (twofold) with a concomitant reduction in the percentage of cells in Go→G1 and S phases, suggesting impaired cell cycle progression (Figure 2B). In addition, we observed that expression of miR-17∼92 sponge increased apoptosis compared with control vector in the Karpas 299 cell line (Figure 2C). Similar results were obtained with the JB6 cell line (data not shown). Since STAT3 is a major downstream substrate for ALK, we further examined the miR-17∼92 expression level upon inhibition of STAT3 phosphorylation by the small-molecule inhibitor Stattic (Calbiochem). The 2 representative members of this cluster, miR-19a and miR-92, were measured by qRT-PCR in Karpas 299 cells, and decreased expression was observed at 2 µM Stattic compared with untreated cells (Figure 2D, upper panel). However, there was little effect on STAT3 phosphorylation at concentrations up to 2 μM (Figure 2D, lower panel) when marked toxicity was observed. Interestingly, miR-17∼92 inhibition (by sponge construct) in the Karpas 299 cell line combined with Stattic at sub-STAT3 inhibitory doses (1.5 µM concentration) showed enhanced cytotoxicity (Figure 2E), suggesting that miR-17∼92 is crucial for cell survival, particularly in the presence of toxic agents. Even though PTEN is a canonical miR-17∼92 target, we did not observe a consistent increase of PTEN protein expression or changes in the activation of AKT pathway in sponge-transduced cells (Figure 2F). The ALK-associated miRNA signature included several other miRNAs that could target PTEN, such as miR-22, miR-106b-25, miR-221, and miR-22237 ; miR-17∼92 may act mainly through some other targets.
KD of miR-17∼92 in ALK(+) ALCL cell lines. (A) Diagram of the lentivirus construct expressing GFP and a sponge that has 7 tandem binding sites for each of the 4 miRNA families present in the miR-17∼92 cluster (targeting from 5′ to 3′, miR-17, miR-20, miR-18, miR-19, and miR-92). (B) Cell cycle analysis. (C) Apoptosis analysis in Karpas 299 after miR-17∼92 KD. (D) Relative expression of 2 representative miR17∼92 cluster members (miR-19a and miR-92) after treatment with Stattic for 12 hours (upper panel). pYSTAT3 levels after treatment with various concentrations of Stattic in ALCL cell lines (Karpas 299, JB6, L82). (E) Inhibitory concentration 50% (IC50) for the STAT3 inhibitor Stattic in Karpas 299 cells (right panel) and combined effect of Stattic and miR-17∼92 inhibition in Karpas 299 (left panel). (F) Immunoblotting of PTEN, pYSTAT3, STAT3 (total), pAKT, and pRPS6 with or without miR-17∼92 KD. DMSO, dimethylsulfoxide.
KD of miR-17∼92 in ALK(+) ALCL cell lines. (A) Diagram of the lentivirus construct expressing GFP and a sponge that has 7 tandem binding sites for each of the 4 miRNA families present in the miR-17∼92 cluster (targeting from 5′ to 3′, miR-17, miR-20, miR-18, miR-19, and miR-92). (B) Cell cycle analysis. (C) Apoptosis analysis in Karpas 299 after miR-17∼92 KD. (D) Relative expression of 2 representative miR17∼92 cluster members (miR-19a and miR-92) after treatment with Stattic for 12 hours (upper panel). pYSTAT3 levels after treatment with various concentrations of Stattic in ALCL cell lines (Karpas 299, JB6, L82). (E) Inhibitory concentration 50% (IC50) for the STAT3 inhibitor Stattic in Karpas 299 cells (right panel) and combined effect of Stattic and miR-17∼92 inhibition in Karpas 299 (left panel). (F) Immunoblotting of PTEN, pYSTAT3, STAT3 (total), pAKT, and pRPS6 with or without miR-17∼92 KD. DMSO, dimethylsulfoxide.
Diagnostic miRNA expression signature for ALCL
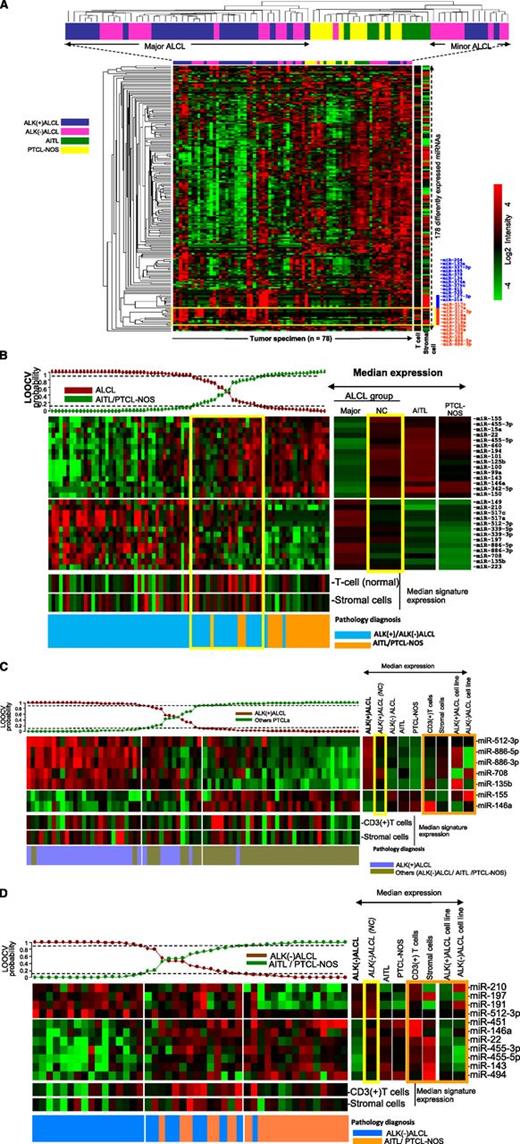
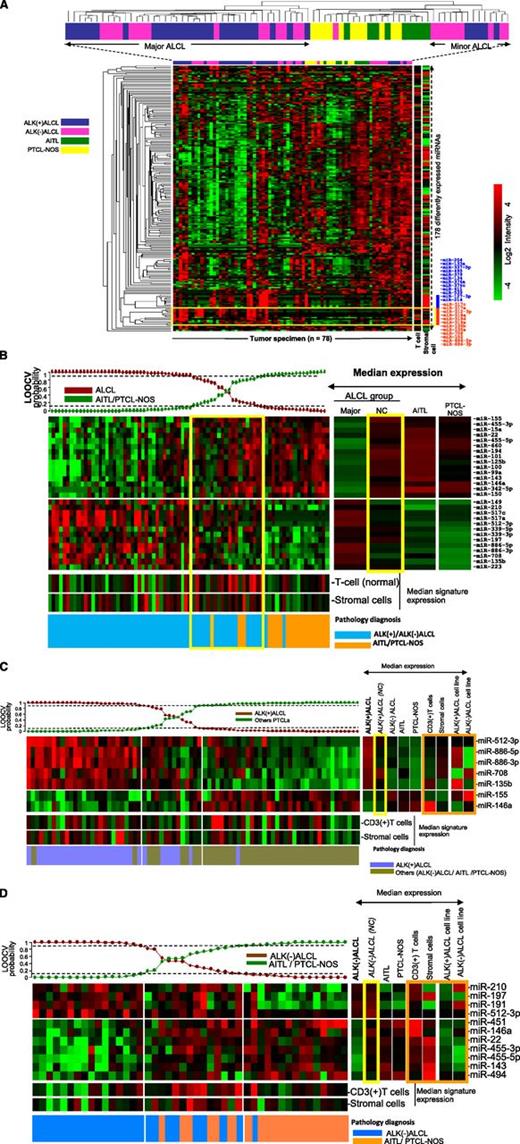
Unsupervised hierarchical clustering (HC) of all PTCL cases (n = 78) showed that ALCL samples formed distinct clusters with respect to PTCL-NOS and AITL, with 2 subgroups of ALCL interspersed among each other (Figure 3A). ALCL cases (n = 58) formed 2 distinct clusters, a major (n = 44) and a minor (n = 14) cluster, with the latter clustering closer to other PTCLs. Of the 178 differentially expressed miRNAs, the majority showed lower expression in ALCL cases compared with other PTCLs. However, a subset of miRNAs (miR-517c, miR-517a, miR-512-3p, miR-135b, miR-135a, miR-708, miR-886-5p, and miR-886-3p) were more highly expressed in ALCL than in other PTCLs (Figure 3A, red bar). There is also a cluster of miRNAs that is highly expressed in some ALCL cases (blue bar). This cluster is also highly expressed in stromal cells, and their expression levels may be related to the stromal content. To identify the miRNA signature specific for ALCL cases, we applied a Bayesian algorithm using ALCL as a group vs other PTCLs and identified 28 miRNAs, including 13 upregulated and 15 downregulated miRNAs to distinguish ALCLs from other PTCLs (Figure 3B). Note that none of the very highly expressed miRNAs are part of the stromal signature, indicating that the stromal component does not have a significant impact in the diagnostic signature. This signature was predictive of most cases in the major ALCL HC (90%; 40 of 44) with >90% probability, whereas a small subset of 9 ALCL cases showed probability of >70% to 90% (6 ALK(+) ALCLs and 3 ALK(–) ALCLs), and 7 ALK(–) ALCLs were below 70%. Two ALK(–) ALCL cases were classified as PTCLs (probability >90%). The majority of ALCL cases, which were not classified as ALCL by the Bayesian algorithm, were part of the minor ALCL cluster (86%; 12 of 14) in an unsupervised hierarchical clustering analysis. This subset of ALCL cases shared many of the 15 miRNAs more highly expressed in PTCL-NOS and AITL.
Unsupervised HC of PTCL cases. (A) ALCL cases formed 2 distinct clusters (major and minor HC clusters) with respect to AITLs and PTCL-NOS. ALK(+) ALCL and ALK(–) ALCL cases were interspersed with each other. A group of miRNAs highly expressed in ALCL cases is highlighted (yellow box) and listed on the right side (red bar). A strong stromal signature is represented by the blue bar. (B) miRNA signature associated with ALCL (vs AITL and PTCL-NOS). The Bayesian classifier included 28 miRNAs. Most of the cases in the ALCL minor hierarchical cluster were in the intermediate group. Yellow boxes highlight the minor ALCL group. (C) miRNA signature associated with ALK(+) ALCL. A 7-miRNA classifier was derived by using the Bayesian algorithm, and it included 5 upregulated and 2 downregulated miRNAs. Orange box highlights the expression of this signature among CD3+ normal T cells, stromal cells, and ALCL cell lines. (D) miRNA signature associated with ALK(–) ALCL. An 11-miRNA classifier included 4 upregulated and 7 downregulated miRNAs. Orange box highlights the comparative analysis of this signature in CD3+ normal T cells, stromal cells, and ALCL cell lines. LOOCV, leave-one-out-cross validation; NC, not classifiable.
Unsupervised HC of PTCL cases. (A) ALCL cases formed 2 distinct clusters (major and minor HC clusters) with respect to AITLs and PTCL-NOS. ALK(+) ALCL and ALK(–) ALCL cases were interspersed with each other. A group of miRNAs highly expressed in ALCL cases is highlighted (yellow box) and listed on the right side (red bar). A strong stromal signature is represented by the blue bar. (B) miRNA signature associated with ALCL (vs AITL and PTCL-NOS). The Bayesian classifier included 28 miRNAs. Most of the cases in the ALCL minor hierarchical cluster were in the intermediate group. Yellow boxes highlight the minor ALCL group. (C) miRNA signature associated with ALK(+) ALCL. A 7-miRNA classifier was derived by using the Bayesian algorithm, and it included 5 upregulated and 2 downregulated miRNAs. Orange box highlights the expression of this signature among CD3+ normal T cells, stromal cells, and ALCL cell lines. (D) miRNA signature associated with ALK(–) ALCL. An 11-miRNA classifier included 4 upregulated and 7 downregulated miRNAs. Orange box highlights the comparative analysis of this signature in CD3+ normal T cells, stromal cells, and ALCL cell lines. LOOCV, leave-one-out-cross validation; NC, not classifiable.
Diagnostic miRNA expression signature for ALK(+) ALCL
According to the 2008 WHO classification,1 ALK(+) ALCL and ALK(–) ALCL were designated as different entities. We observed 7 miRNAs, including 5 upregulated miRNAs (miR-512-3p, miR-886-5p, miR-886-3p, miR-708, and miR-135b) and 2 downregulated miRNAs (miR-155 and miR-146a) differentially expressed in ALK(+) ALCLs compared with ALK(–) ALCLs and other PTCLs (Figure 3C). Interestingly, 2 ALK(–) ALCL cases were classified as ALK(+) ALCLs with this miRNA signature. The class precision analysis indicated that 25 of 33 ALK(+) ALCLs were classified with >90% probability, and a subset of ALK(+) ALCL cases (n = 7) was unclassifiable with probabilities of 60% to 90% (n = 3) and <50% (n = 4). One ALK(+) ALCL case was classified as other PTCL. This unclassifiable subset of cases showed no major difference in morphology, tumor content (% neoplastic cells), or T-cell and stromal cell miRNA signature, but patients in this unclassifiable group were relatively older (median age, 38 years; range, 21 to 71 years) compared with the molecularly classified cases (median age, 15 years; range, 2 to 62 years), though the difference was not statistically significant.
Other than the miRNAs in the classifier, we analyzed the differential expression of miRNAs between molecularly defined ALK(+) ALCL cases and other PTCLs (P < .05 and false recovery rate < 0.1) and observed that 22 miRNAs, including the chr19q13.41-42 miRNA (C19MC) cluster (miR-519a, miR-517c, and miR-517d), were upregulated in ALK(+) ALCL. A small subset of these miRNAs may be contributed by the stromal cells (eg, miR-221, miR-376a, miR-134, miR-495, and miR-379) (supplemental Figure 3a). Of the 17 downregulated miRNAs, aside from miRNA-146a and miR-155, several other interesting miRNAs were noted, including miR-150, miR-101, miR-342-3p, miR-342-5p, miR-142-3p, and miR-142-5p. This group of miRNAs was also highly expressed in normal T cells.
miRNA signature for differentiating ALK(–) ALCLs from PTCLs
Using an approach similar to the one above, we obtained an 11-miRNA signature, including 4 upregulated (miR-210, miR-197, miR-191, and miR-512-3p) and 7 downregulated (miR-451, miR-146a, miR-22, miR-455-3p, miR-455-5p, miR-143, and miR-494) miRNAs, which distinguished ALK(–) ALCL cases (16 of 25) with >90% probability (Figure 3D). However, of the remaining 9 cases, 3 with probability <50% and 1 with probability <10% were classified as PTCLs. We observed no significant differences in tumor content or morphology between these 2 subsets. Interestingly, the 7 downregulated miRNAs in the classifier were highly expressed in T cells or stromal cells.
We further compared the differential miRNA expression between molecularly defined ALK(–) ALCLs and other PTCLs (P < .05; false recovery rate < 0.1), and observed 47 miRNAs differentially expressed, including upregulation of miR-210, miR-512-3p, miR-135b, miR-191, and miR-886-3p, and many downregulated miRNAs, including miR-451, miR-22, miR-146a, and miR-101, in ALK(–) ALCLs (supplemental Figure 3b).
We also compared the miRNA profiles of ALK(–) ALCLs and ALK(+) ALCLs and observed 43 differentially expressed miRNAs, which included 27 highly expressed miRNAs in ALK(+) ALCLs and 16 miRNAs more highly expressed by ALK(–) ALCLs (supplemental Figure 3c). Many of these miRNAs were common to those observed to be differentially expressed between ALK(+) ALCL and other T-cell lymphomas.
Discussion
We included a relatively large series of well-characterized PTCL cases with the aim of determining distinct patterns of miRNA expression for the ALCLs and to further subdivide ALK(–) ALCL from AITL and PTCL-NOS. We were able to construct miRNA classifiers that can distinguish the majority of ALCL entities, and we also identified several miRNAs that may play a role in the pathogenesis of ALCL. One interesting observation is that while many T-cell–associated genes are downregulated in ALCLs, many T-cell associated miRNAs are still expressed, indicating the preservation of the cell of origin miRNA expression profile.
The known molecular mechanism of the pathogenesis of ALK(+) ALCL involves activation of STAT3, a major substrate of ALK, which is required for the survival and growth of ALK(+) ALCL cells in vivo and in vitro.3,6 We performed in vitro experiments to identify miRNAs that are associated with ALK activation and observed that the miR-17∼92 cluster and its paralogues were downregulated on ALK KD. It is known that the miR-17∼92 cluster is transcriptionally regulated by STAT3, suggesting a role of this miRNA cluster in the pathogenesis of ALK(+) ALCL. Consistent with this possibility, we observed that miR-17∼92 is generally highly expressed in ALK(+) ALCL, and KD of this miRNA cluster did have deleterious effects on cell survival and proliferation. We were not able to demonstrate the regulation of miR-17∼92 expression by pYSTAT3 using the small-molecular inhibitor Stattic because it was highly toxic to the ALCL cells before clear STAT3 inhibition was observed. Interestingly, inhibition of miR17∼92 by the sponge construct enhanced the toxicity of Stattic. Thus, the miRNA cluster is probably important in promoting survival of ALCL cells, especially when they are under stress. However, one critical target of miR-17∼92, PTEN, showed only mild upregulation on reducing the level of the miRNA cluster,37 and there were little changes in pAKT, suggesting that the effect of miR17∼92 may be exerted through other targets. There were only a limited number of additional miRNAs identified by the ALK KD experiments that have the expected pattern in tumor samples. These included miR-93, miR-100, miR-629, and miR-886-5p. The lack of positive correlation between experiments performed in cell lines and the observations in ALK(+) ALCL cases suggested complex regulation of the miRNAs in vivo, the presence of various stromal components in tissue specimens that contributed to the variability of the miRNA expression profile, or alterations specific to the cell lines.
The pathognomonic feature of ALK(+) ALCL can be determined readily by IHC and gene rearrangement of ALK. However, we identified 7 miRNAs—5 upregulated (miR-512-3p, miR-886-5p, miR-886-3p, miR-708, and miR-135b) and 2 downregulated (miR-146a and miR-155)—that were a signature characteristic of ALK(+) ALCL cases and that also define some important aspects of ALCL tumor biology. This miRNA signature includes high expression of miR-135b (>15-fold), which has been shown to mediate the NPM-ALK–induced interleukin-17 (IL-17) –producing immunophenotype in ALK(+) ALCL,23 an observation consistent with our gene expression profiling study.13 High expression of miR-886-5p and miR-886-3p may also be related to ALK expression, since ALK KD was associated with significant downregulation of these miRNAs. miR-886 has been shown to target Bax,38 a proapoptotic gene, and thus may deregulate apoptosis. High expression of miR-708 and miR-512-3p in ALK(+) ALCL compared with other PTCLs may be relevant, since miR-708 has been shown to target transmembrane protein 88 (TMEM88), a negative regulator of Wnt signaling,39 and miR-512-3p targets the cell cycle inhibitor p21(Waf1/Cip1).40 Thus, the miRNAs in the signature may contribute to the expression of an IL-17–associated phenotype and inhibition of apoptosis as well as promoting cell cycle progression. However, the function of miRNA on gene expression is cell and microenvironment dependent and therefore must be investigated in a context-specific manner.
miR-155, a known oncomir in DLBCL,41 is expressed at a significantly lower level in ALK(+) ALCL compared with ALK(–) ALCLs and PTCLs. This suggests the use of a different oncogenic pathway. It is noteworthy that the miR-17∼92 cluster is highly expressed in ALK(+) ALCL and possibly may serve as an alternative to miR-155. The low expression of miR-146a is a striking feature of ALK(+) ALCLs. miR-146a is known to regulate innate immunity and is repressed by MYC while it is upregulated by nuclear factor κB (NF-κB) and TCR signaling. Its role in ALCL pathogenesis is unclear, but its low level may be related to the lack of TCR signaling. miR-146a has been shown to target STAT1, and its low expression may lead to increased STAT1 expression.42 Our miRNA signature is distinct from the one derived previously from ALCL cell lines.21 This may be partially attributed to its derivation from tissue specimens and evaluation against PTCL entities. In our experience, cell lines show a distinct miRNA profile from primary tumors with loss of many miRNAs. Although this may be partially due to the lack of stromal components, there are probably changes associated with immortalization in cell culture as well as culture-associated changes. However, a number of our observations are consistent with those of Merkel et al21 such as high expression of the miR-17∼92 cluster and miR-886-3p and low expression of miR-155. The miR-17∼92 cluster is located at 13q31.3, which is frequently amplified in lymphomas and other cancers and is highly expressed in cancer cells.20 It is also induced by MYC and cooperates with MYC in lymphomagenesis.43,44 An autoregulatory loop has been shown between STAT3 and miR-17∼9245 that may be the major mechanism of its upregulation in ALCL.
The distinction of ALK(–) ALCLs from PTCL-NOS can be challenging with conventional diagnostics. The 11-miRNA signature that could distinguish ALK(–) ALCL from PTCL-NOS and AITL (including the upregulation of miR-512-3p and downregulation of miR-146a) was also noted in ALK(+) ALCL, suggesting a shared role of these miRNAs in ALCL biology. The other 3 upregulated miRNAs include (1) miR-210, a regulator of hypoxic responses that has been associated with tumor progression46 ; (2) miR-197, a negative regulator of the tumor suppressor gene FUS1 in lung cancer47 ; and (3) miR-191, which is associated with hepatocellular carcinoma.48 The downregulated miRNAs include miR-451, miR-146a, miR-22, miR-455-3p, and miR146, as noted above. miR-2249 and miR-45150 are reportedly associated with tumor suppressor activities.
Other than miRNAs in the classifier, we observed 35 miRNAs differentially expressed between ALK(–) ALCLs and PTCLs, with 21 upregulated and 14 downregulated miRNAs, including miR-886-3p and miR-886-5p, whose expression was observed in both types of ALCL, although it was higher in ALK(+) cases. Two miRNAs, miR-29b and miR-29a, may be deregulated by recurrent t(6;7)(p25.3;q32.3) translocation in ALK(–) ALCLs,19 but only miR-29b was upregulated in ALK(–) ALCL compared with other PTCLs and ALK(+) ALCL.
Another major aim of this study was to compare ALK(+) ALCLs and ALK(–) ALCLs; however, analysis of unsupervised HC had shown that ALCL entities were interspersed regardless of ALK expression status, and the top 10% of expressed miRNAs in ALK(–) ALCLs and ALK(+) ALCLs showed a significant overlap (94.7%; 36 of 38), suggesting that ALK(+) ALCL and ALK(–) ALCL share common miRNA profiles. There was a set of miRNAs expressed by ALCL cases, regardless of their ALK expression (miR-517c, miR-517a, miR-518e, miR-519d, miR-519a, miR-135a, and miR-152) when compared with other PTCLs. However, there were also significant differences between the 2 entities (supplemental Figure 3c). Consistent with previous reports, miR-29b, miR-29a,24 and miR-16,22 which have been shown to be downregulated by ALK, were also expressed at lower levels in ALK(+) ALCL cases in our series.
One notable observation was that there was a minor HC cluster that included both subtypes of ALCL that accounted for most of the cases that could not be classified molecularly. For the minor ALK(+) ALCL subset, there tended to be lower expression of miRNAs associated with ALCL but at the same time, the expression of miRNAs associated with PTCLs was also low. It is possible that these cases just had low tumor cell content, but the stromal signature was not high, and review of the sections did not show an obviously lower number of neoplastic cells or distinct morphological features. The minor subset of the ALK(–) ALCL cases actually showed a higher expression of the ALCL signature compared with the major subset but it also had a stronger expression of the PTCL-associated miRNAs. It is possible that this reflects a higher infiltration of T cells, but one would anticipate a decrease in the ALCL signature and an increase in the stromal signature; however, that was not the case. Review of the section showed consistent morphological features of ALCL and also a substantial number of neoplastic cells. In fact, these minor groups of ALCL cases in general expressed a set of miRNAs common to all ALCLs.
Overall, our study confirmed that miRNA signatures can be used as a diagnostic tool for ALCLs and can play a significant role in their pathogenesis. We have also shown that ALK– ALCL has a distinct miRNA profile compared with PTCL-NOS and partially shares the miRNA expression profile of ALK(+) ALCL. However a larger series of cases would be required to further refine the diagnostic signatures and to elucidate the biologic differences between the major and minor subsets of ALCL cases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Martin Bast and Xin Huang for coordinating clinical outcome data and analysis.
This work was supported in part by a grant from the National Cancer Institute (5U01/CA114778) (W.C.C.).
Authorship
Contribution: C.L., J.I., G.I., and W.C.C. designed the experiments, analyzed the data, and wrote the manuscript; Y.S. and M.J.D. performed experiments; K.D. provided support to M.J.D. and helped to write the manuscript; J.I., J.T.-F., M.S.L., S.B., T.C.G., D.D.W., P.A., S.L.P., and W.C.C. provided materials and performed pathology review; and A.B., E.P., E.S., C.M.L., C.J., R.P., C.K., and X.H. performed in vitro experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wing C. Chan, Center for Research in Lymphoma and Leukemia, Department of Pathology and Microbiology, 983135 Nebraska Medical Center, Omaha, NE 68198-3135; e-mail: jchan@unmc.edu.
References
Author notes
C.L. and J.I. contributed equally to this work.