Key Points
Germline JAK2V617I mutation as a sole genetic event does not suppress hematopoietic stem cells.
JAK2V617I induces weaker constitutive activation than JAK2V617F but considerable cytokine hyperresponsiveness.
The association between somatic JAK2 mutation and myeloproliferative neoplasms (MPNs) is now well established. However, because JAK2 mutations are associated with heterogeneous clinical phenotypes and often occur as secondary genetic events, some aspects of JAK2 mutation biology remain to be understood. We recently described a germline JAK2V617I mutation in a family with hereditary thrombocytosis and herein characterize the hematopoietic and signaling impact of JAK2V617I. Through targeted sequencing of MPN-associated mutations, exome sequencing, and clonality analysis, we demonstrate that JAK2V617I is likely to be the sole driver mutation in JAK2V617I-positive individuals with thrombocytosis. Phenotypic hematopoietic stem cells (HSCs) were increased in the blood and bone marrow of JAK2V617I-positive individuals and were sustained at higher levels than controls after xenotransplantation. In signaling and transcriptional assays, JAK2V617I demonstrated more activity than wild-type JAK2 but substantially less than JAK2V617F. After cytokine stimulation, JAK2V617I resulted in markedly increased downstream signaling compared with wild-type JAK2 and comparable with JAK2V617F. These findings demonstrate that JAK2V617I induces sufficient cytokine hyperresponsiveness in the absence of other molecular events to induce a homogeneous MPN-like phenotype. We also provide evidence that the JAK2V617I mutation may expand the HSC pool, providing insights into both JAK2 mutation biology and MPN disease pathogenesis.
Introduction
It is now well-established that acquisition of a somatic JAK2 mutation has a fundamental role in the pathogenesis of Philadelphia-negative myeloproliferative neoplasms (MPNs). This is supported by the remarkably frequent occurrence of JAK2 mutation in MPNs,1,-3 as well as evidence that this mutation causes constitutive activation of the JAK2 kinase in cell lines1 and recapitulates features of MPNs in multiple mouse models.4 Nevertheless, a number of outstanding questions with regard to the role of JAK2 mutation in MPN pathogenesis remain. First, it has been proposed that mutations in JAK2, or other signaling molecules, may require additional molecular events to induce a fully penetrant hematopoietic disease.3 This is important because the JAK2V617F mutation occurs in a wide variety of different MPN phenotypes,5 myelodysplasia,6 and acute myeloid leukemia,7 as well as in patients without any hematopoietic phenotype,8 and this heterogeneity may in part relate to the presence of other molecular events or genetic modifiers.3,5 Indeed, a number of lines of evidence support that JAK2 mutation frequently occurs as a late event in MPN pathogenesis. First, the JAK2 mutation is often only present in a subclone at diagnosis, despite evidence of clonality by X-chromosome inactivation patterns (XCIP) or cytogenetic studies.9,-11 Second, transformation to acute leukemia from prior JAK2V617F-positive MPN is often JAK2 wild-type.12 Finally, in familial MPNs, clonal hematopoiesis is often observed,13 and occurrence of JAK2V617F is heterogeneous and occurs as a somatic rather than a germline mutation in association with the MPN phenotype.14 Thus, the underlying inherited genetic abnormality in the majority of these families remains unknown.15,16 However, although these observations support that JAK2 mutation occurs as a late event in certain cases of MPN, they do not exclude the possibility that JAK2 mutation might also occur as an initiating and perhaps the only genetic event in other cases.3 Thus, it remains uncertain whether JAK2 mutation occurs as an initiating event and, if so, whether JAK2 mutation alone is sufficient for the development of human disease.
A second unresolved issue in the understanding of the role of JAK2 mutations in MPN pathogenesis relates to the impact of an isolated JAK2 mutation on hematopoietic progenitors. In sporadic MPN, JAK2 mutation occurs as a somatic event in a single cell, which then undergoes clonal expansion. Studies of human MPN17 and mouse models of JAK2V617F4 have suggested that the JAK2 mutant positive disease–initiating cell resides within the phenotypic hematopoietic stem cell (HSC) compartment. However, the impact of JAK2 mutation on HSCs is controversial. Some studies have suggested normal numbers of HSCs in patients with JAK2V617F polycythemia vera (PV) and essential thrombocythemia (ET),18 whereas other studies have suggested an expansion of HSCs in PV.17
Mouse models of JAK2V617F expressed from the endogenous genetic loci have provided important insights into JAK2 mutation biology. However, different models of the JAK2V617F mutation have resulted in strikingly heterogeneous phenotypes, with mice primarily developing an essential thrombocythemia phenotype in one model19 as opposed to polycythemia in others,20,-22 and a phenotype that varied according to the ratio of mutant JAK2V617F to wild-type in another model.23 Moreover, development of myelofibrosis also varied markedly.19,,,-23 Furthermore, these different models have produced conflicting results with regards to the impact of JAK2V617F on hematopoietic stem and progenitor cells, with different models suggesting a suppression,19 no impact,20 or expansion of HSCs21,24 by the JAK2V617F mutation. One proposed explanation for this heterogeneity is that it relates to expression of human19 versus murine20,-22 forms of the JAK2 protein.4 However, understanding the impact of JAK2 mutation on human hematopoiesis is difficult in MPNs in which the JAK2 mutation may be present in only a subclone that may also have acquired multiple additional and heterogeneous genetic hits.
We have recently for the first time identified a family with germline JAK2V617I mutation associated with high-penetrance hereditary thrombocytosis.25 Furthermore, additional preliminary reports have identified other germline JAK2 mutations in patients with myeloproliferative phenotype.26,-28 In the present studies, we further explored whether the JAK2V617I mutation is sufficient to induce hematopoietic disease as a sole genetic event. We have also characterized the hematopoietic stem and progenitor cell phenotype of patients carrying the germline JAK2V617I mutation to gain insights into the impact of an isolated JAK2 mutation on human hematopoiesis. Finally, we compared the signaling consequences of JAK2V617I with the more common JAK2V617F mutation.
Methods
Patients and samples
Patients provided written informed consent in accordance with the Declaration of Helsinki for sample collection and use in research under Oxford University ethics committee approval. Peripheral blood (PB) mononuclear cells (MNCs) were isolated by density gradient centrifugation (Lymphoprep, Axis-Shields, Oslo, Norway) and in all cases were further analyzed directly. Four cases carrying germline JAK2V617I mutation were studied. Clinical details have been reported previously.25 In brief, at the time of sampling, case 1 (C1) was a 59-year-old woman who presented at the age of 53 with a cerebrovascular event associated with thrombocytosis (750 × 109/L). She was treated as a JAK2-negative case of ET and had received hydroxycarbamide therapy for 6 years before the current study. The daughter of C1 (C2, aged 34) and two sons C3 and C4, aged 36 and 38, respectively, all had a mild (451 to 648 × 109/L) asymptomatic thrombocytosis and had not received cytoreductive therapy, whereas C5, the daughter of C1 (aged 40 years), had a normal platelet count and was JAKV617I-negative, and samples were used as an additional negative control for PB analysis. Nine patients with classical MPNs were also studied. Clinical details of these patients are summarized in supplemental Table 1.
EZH2, CBL, TET2, NPM1, FLT3 ITD, and D835 mutation screening
ASXL1 mutation screening
Exon 12 of the ASXL1 gene was amplified by polymerase chain reaction using previously described primers.34 Products were then analyzed by Sanger sequencing using the BigDye Terminator v3.1 Cycle Sequencing kit, with both the forward and reverse primers, according to the manufacturer’s protocol.
IDH1, IDH2, KIT, and DNMT3a mutation screening
Previously described primers35 were used to amplify a 251bp region surrounding IDH1 codon 132, and a 256bp region surrounding both IDH2 codons 140 and 172. For KIT, previously described primers36 were used to amplify a 113bp region surrounding KIT codon 816. DNMT3a R882 primers were designed using the Primer3 program (primer sequences available on request).37 In each case the 5′ end of a single primer was biotinylated and subject to pyrosequencing previously described,38 with the appropriate dispensation order to interrogate the target codon (supplemental Table 2).
XCIP clonality analysis
XCIP clonality analysis was performed as described previously.9
Whole-exome sequencing
Five µg of PB and germline (CD3-selected T-cells; from C2 only) genomic DNA was obtained from the index patients (C1 and C2). Shotgun libraries were prepared, followed by “in-solution” whole-exome capture using the SureSelect Human All Exon v.4 kit (Agilent Technologies, CA). The sample was indexed, and paired-end massively parallel sequencing was performed on either a single (germline) lane or paired (PB) lanes of the Illumina HiSeq2000 platform, generating 19 MB of data. The sequence reads were mapped to the human genome assembly hg19/NCBI37 using Stampy,39 and variants were detected using Platypus (www.well.ox.ac.uk/platypus). Only high-quality calls (score >200) were included in the analysis. For C2, germline variants in T cells were subtracted from variants occurring in myeloid cells. We excluded variants located in noncoding regions, those predicted to be benign by in silico analysis (SIFT, sift.jcvi.org/; PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/), and variants that were described as nonpathogenic in dbSNP137 and that in addition had no reference to myelodysplasia, MPN, or acute myeloid leukemia in the Catalogue of Somatic Mutations in Cancer.
Flow cytometric analysis of PB and BM stem and progenitor cells
For analysis of hematopoietic stem and progenitor cells, PB or bone marrow (BM) MNCs were isolated by Lymphoprep (Nycomed, Oslo, Norway) gradient centrifugation. Ten million MNCs were then washed in ice-cold phosphate-buffered saline (PBS) with 2% fetal calf serum (FCS) and resuspended in human Fc-block (Miltenyi) and incubated on ice for 15 minutes. Cells were then incubated on ice for 15 minutes with optimized concentrations of CD34-allophycocyanin (Becton-Dickinson), CD38-phycoerythrin-Texas red (PETxR) (Invitrogen), CD90-phycoerythrin (PE) (Becton-Dickinson or BioLegend, San Diego, CA), CD45-AF700 (BioLegend), CD123 (IL3Ra)-PECy7 (BioLegend), CD45RA-fluorescein isothiocyanate (Invitrogen), and a lineage cocktail of PECy5-conjugated (CD2, CD3, CD4, CD7, CD8, CD11b, CD19, CD20, and Glycophorin A from BioLegend; CD56 from Becton-Dickinson; CD10 from Caltag; and CD14 from Beckman Coulter) monoclonal antibodies. Cells were then washed in 1 mL of ice-cold PBS with 2% FCS and resuspended in 200 μL of ice-cold PBS with 2% FCS before analysis on an LSR II flow cytometer (BD Biosciences). All experiments included fluorescent-minus-one controls. Progenitor populations were defined as follows: phenotypic HSCs (CD45+Lin–CD34+CD38–CD90+CD45RA–), multipotent progenitors (MPPs; CD45+Lin–CD34+CD38–CD90–CD45RA–), common myeloid progenitors (CMP; CD45+Lin–CD34+CD38+CD123+CD45RA–), granulocyte macrophage progenitors (GMPs; CD45+Lin–CD34+CD38+CD123+CD45RA+), and megakaryocyte erythroid progenitors (MEPs; CD45+Lin–CD34+CD38+CD123–CD45RA−).40
Colony assays
For colony-forming unit (CFU)-GM, burst-forming unit (BFU)-E, and CFU-GEMM, MethoCult H4434 Classic (Stem Cell technologies, Grenoble, France) was used according to the manufacturer’s recommended conditions with 15 000 MNCs plated per 1 mL.
For granulocyte colony-stimulating factor (G-CSF) and thrombopoietin (TPO) cytokine response colony assays, 15 000 BM MNCs or 15 000 to 60 000 PB MNCs (depending on numbers of circulating progenitors by flow cytometry) were plated in methylcellulose (H4100; Stem Cell Technologies) supplemented with FCS (Stem Cell Technologies), 100 U/mL penicillin/streptomycin, 2 mM l-glutamine, 0.1 mM 2-βmercaptoethanol, and human (rh) stem cell factor (SCF; Amgen, Thousand Oaks, CA) at 50 ng/mL. To assess colony formation under conditions of low cytokine concentrations, either rhG-CSF (Amgen) or rhTPO were added at 2 different concentrations (100 ng/mL or 0.8 ng/mL). For evaluation of generation of cytokine-independent colonies, 15 000 BM or 200 000 PB MNCs were plated as stated before, but in the absence of cytokines. For erythropoietin (EPO) cytokine response assay, 50 000 BM MNCs or 100 000 PB MNCs were plated in methylcellulose (H4100; Stem Cell Technologies) in the presence of EPO at 5 U/mL, 0.05 U/mL, and 0.005 U/mL and no EPO. CFU-GM and BFU-E were counted after 2 weeks.
For cytokine-dependent CFU-Mk assays, 100 000 BM MNCs were plated using the MegaCult-C kit according to the manufacturer’s recommended conditions (Stem Cell Technologies). Final cytokine concentrations were rhTPO 50 ng/mL (Genentech), rhIL-3 10 ng/mL, and rhIL-6 10 ng/mL.
Xenotransplant assay
NOD/LtSz-scidIL2Rgnull (NSG) mice (Jackson Laboratory) were bred and housed at the University of Oxford animal care facility. Ten- to 14-week-old mice were sublethally irradiated with 125 cGy twice 4 hours apart. CD34+ cells (1 × 105) were resuspended in PBS supplemented with 1% FCS and injected via the tail vein 4 to 24 hours after the last irradiation dose. In C1 to C3, CD34 selection resulted in CD34 purity of 83% to 89%, and 2 to 3 recipient mice were transplanted per sample. Cell numbers were severely limited in the case of C4, and CD34 selection was suboptimal (36.7% CD34 purity after selection); consequently, only one mouse was transplanted. CD34 purity was >90% for both normal BM (NBM) controls, and each was transplanted into 2 recipient mice. After 5 and 10 weeks posttransplantation, BM was aspirated from mice and analyzed for human multilineage engraftment, defined as human CD45 engraftment above 0.1% of total CD45+ cells within NSG BM with both lymphoid (CD19+) and myeloid (CD15/CD33/CD66b+) cells present. All experiments were conducted in accordance with UK Home Office–approved Project License 30/2570.
For monitoring engraftment, BM aspirates were lysed in ammonium chloride. Nonspecific antibody binding was blocked by preincubation in both human (Miltenyil Biotech) and mouse Fc-blocking reagents. Cells were stained with anti-human CD45-AF700 (clone HI30, Biolegend), CD19-PE (clone SJ25C1, BD), CD15-FITC (clone MMA, BD), CD33-FITC (clone P67.6, BD), CD66b-FITC (clone G10F5, BD), and CD34-allophycocyanin (clone 8G12, BD), and anti-mouse CD45-Pacific Orange (clone 30-F11, Invitrogen). Progenitor cell staging was as for analysis of primary BM samples with the addition of anti-mouse CD45-Pacific Orange (clone 30-F11, Invitrogen). Viable cells were visualized by exclusion of 7-aminoactinomycin D (Invitrogen) and analyzed on fluorescence-activated cell sorting LSRII (Beckton Dickinson).
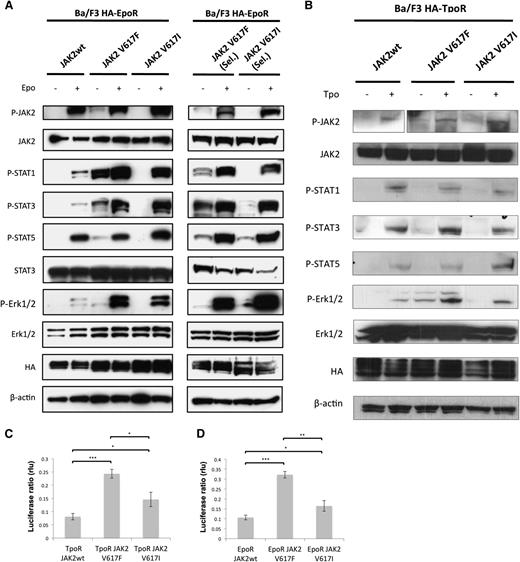
Signaling by JAK2-V617I versus JAK2-V617F in hematopoietic Ba/F3 cells
Ba/F3 cells expressing murine EpoR (Ba/F3 EpoR) or TpoR (Ba/F3 TpoR) were transduced with bicistronic retroviruses coding for JAK2 wild-type, JAK2V617F or JAK2V617I. Sorting for cell surface CD4 (the marker downstream of the internal ribosomal entry site in the bicistronic retroviruses) was applied to ensure equivalent JAK2 expression among the 3 constructs; these levels were approximately fivefold higher than Ba/F3 cell endogenous JAK2 levels. Cells were maintained in RPMI medium supplemented with 10% fetal bovine serum and IL-3 derived from supernatant of WEHI cells (equivalent to 2 ng/mL IL3). Cells maintained in IL3 (denoted sorted) were washed 4 times and cultivated in medium without IL3. Only cells expressing JAK2V617F and JAK2V617I acquired IL-3 independence, as described,41 although JAK2V617I cells were slower to become autonomous. Sorted cells grown in the presence of growth factor and those selected for factor independence were then starved from serum and cytokines for 5 hours and lysed in 1% NP40 lysis buffer (containing 100 μM Na orthovanadate, 100 μM phenylmethylsulfonyl fluoride, and a cocktail of protease inhibitors), as described42 and processed for Western blotting with antibodies against JAK2, STAT3, Erk1/2, or against phosphosites in these proteins that reflect activation.43 The sources of antibodies were: anti-HA (Roche Diagnostics, Indianapolis, IN), anti-JAK2 (Santa Cruz, anti-actin, Sigma, St. Louis, MO), anti–phospho-JAK2 (Tyr1007/1008), anti–phospho-STAT1 (Tyr701), anti–phospho-STAT3 (Tyr705), anti–phospho-STAT5 (Tyr694), anti-phospho–extracellular signal–regulated kinase 1/2 (Erk1/2; Tyr202/204), and antibodies recognizing Erk1/2 were obtained from Cell Signaling Technology Inc. (Danvers, MA).
Dual luciferase assays
γ2A cells, which are fibrosarcoma cells devoid of JAK2, were transfected with the cDNAs of TpoR or EpoR, along with wild-type JAK2, JAK2V617F, or JAK2V617I; STAT5 cDNAs and reporter firefly pGRR5-luc (a reporter for STAT5 transcriptional activation); and renilla luciferase constructs, as described before.44 Cells were lysed 24 hours after transfection and assessed for firefly and renilla luciferase activity.
Statistical analysis
All statistical analyses were carried out using the unpaired Student t test, and results are indicated when P < .05.
Results
Absence of clonality and MPN-associated mutations in JAK2V617I cases
We first investigated the possibility of secondary somatic genetic events in patients with germline JAK2V617I mutation. To determine whether patients with germline JAK2V617I had evidence of clonal hematopoiesis, we carried out XCIP clonality analysis on the 2 potentially informative female cases (C1 and C2). C2, a young woman with mild, asymptomatic thrombocytosis, showed polyclonal hematopoiesis (59% predominant allele in both T cells and myeloid cells), strongly supporting that the thrombocytosis had developed independently of additional somatic events. Clonality testing on C1, an older woman who had received hydroxycarbamide therapy for 6 years, showed a predominant allele of 50% in T cells and 80% in myeloid cells, an inconclusive result given the patient age and borderline allele frequencies. Within the limitations of the assay, this suggests that secondary somatic events with clonal selection have not occurred.
We previously reported absence of acquired copy number abnormalities and/or areas of uniparental disomy as well as lack of mutation of MPL and THPO in JAK2V617I-positive patients, including patients analyzed here.25 To exclude other chronic MPN and blast-phase–associated mutations, we also screened for mutations in IDH1, IDH2, KIT, ASXL1, DMNT3A, TET2, CBL, NPM1, FLT3, and EZH, but no mutations were detected in these genes in the 4 cases we screened. Because this targeted sequencing would not detect other known MPN-associated mutations, we also carried out exome sequencing on 2 JAK2V617I-positive cases: C1, the case with the most marked phenotype and an inconclusive clonality analysis, and C2, who had paired T-cell DNA available as a germline control. None of the variants detected in C1 or C2 has been reported to occur in chronic or blast-phase MPNs using the filters described in Methods.45
Together with the evidence of polyclonal hematopoiesis, and high-penetrance clinical phenotype, these data support that JAK2V617I is likely to be the sole genetic abnormality, sufficient to induce the MPN-like thrombocytosis phenotype observed in patients with germline JAK2V617I mutation.
Hematopoietic stem and progenitor phenotype in JAK2V617I cases
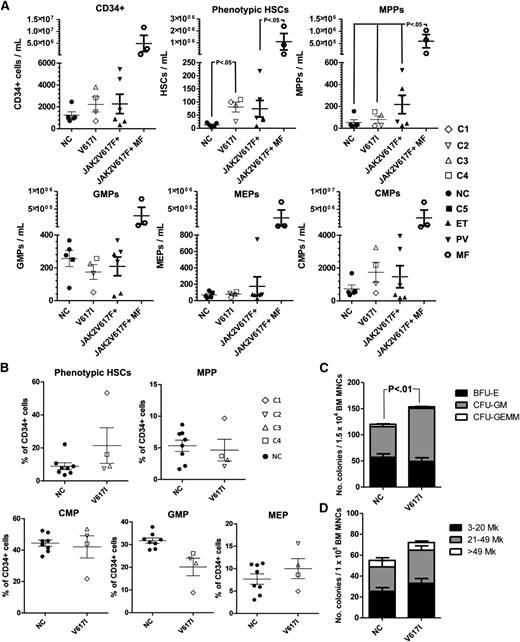
We next investigated the impact of germline JAK2V617I mutation on primitive hematopoiesis. Previous studies have demonstrated an increase in circulating HSCs in the PB of patients with normal white blood cell counts, JAK2V617F-positive PV.17 We therefore quantified phenotypic (Lin–CD34+CD38–CD90+CD45RA–) HSCs and progenitor populations40 in the PB and BM of 4 individuals with germline JAK2V617I mutation compared with age-matched normal controls (NCs; n = 4) and for PB, also with JAK2V617F-positive cases of MPN (n = 9; Figure 1A and supplemental Figure 1A-B for PB and Figure 1B-D and supplemental Figure 2A-C for BM). Phenotypic HSCs in PB were significantly increased in JAK2V617I-positive patients to a similar level seen in JAK2V617F-positive ET and PV but markedly less than that seen in myelofibrosis (Figure 1A; 6.4 fold increase; P = .03). Similarly, there was a non-significant trend to increased (mean 2.4-fold) numbers of phenotypic HSCs relative to NCs in the BM of JAK2V617I-positive persons (Figure 1B), with a particularly marked increase in phenotypic HSCs in the oldest case studied (C1). There were no significant differences in the numbers of other myelo-erythroid progenitor populations as determined by fluorescence-activated cell sorting, in both PB and BM of JAK2V617I-positive cases (Figure 1A-B). Compared with NCs (n = 4), however, CFU-GM were increased in the BM (Figure 1C; P < .001) of JAK2V617I-positive cases. BFU-Es were not affected, in agreement with the lack of erythroid phenotype in these patients, whereas CFU-Mks were slightly, but nonsignificantly increased in the BM (Figure 1D).
PB and BM hematopoietic phenotype of germline JAKV617I. (A) Quantification of Lin-CD34+, phenotypic HSCs (CD45+Lin–CD34+CD38–CD90+CD45RA–), multipotent progenitors (MPPs; CD45+Lin–CD34+CD38–CD90–CD45RA–), common myeloid progenitors (CMPs; CD45+Lin–CD34+CD38+CD123+CD45RA–), granulocyte macrophage progenitors (GMPs; CD45+Lin–CD34+CD38+CD123+CD45RA+), and megakaryocyte erythroid progenitors (MEPs; CD45+Lin–CD34+CD38+CD123–CD45RA–) in the PB of normal controls (n = 4); JAK2V617I-positive samples (n = 4); patients with JAK2V617F-positive PV (n = 3); ET (n = 3), and myelofibrosis (n = 3). (B) Quantification of phenotypic HSCs, MPPs, CMPs, GMPs, and MEPs in the BM of normal controls (n = 8) and JAK2V617I-positive samples. (C) Numbers of BFU-E, CFU-GM, and CFU-GEMM in the BM of NC (n = 8) and JAK2V617I-positive (n = 4) cases. (D) Numbers and sizes of BM CFU-Mk in NC (n = 3) versus JAK2V617I (n = 4) patients. Error bars represent SEM. P values are shown if < .05.
PB and BM hematopoietic phenotype of germline JAKV617I. (A) Quantification of Lin-CD34+, phenotypic HSCs (CD45+Lin–CD34+CD38–CD90+CD45RA–), multipotent progenitors (MPPs; CD45+Lin–CD34+CD38–CD90–CD45RA–), common myeloid progenitors (CMPs; CD45+Lin–CD34+CD38+CD123+CD45RA–), granulocyte macrophage progenitors (GMPs; CD45+Lin–CD34+CD38+CD123+CD45RA+), and megakaryocyte erythroid progenitors (MEPs; CD45+Lin–CD34+CD38+CD123–CD45RA–) in the PB of normal controls (n = 4); JAK2V617I-positive samples (n = 4); patients with JAK2V617F-positive PV (n = 3); ET (n = 3), and myelofibrosis (n = 3). (B) Quantification of phenotypic HSCs, MPPs, CMPs, GMPs, and MEPs in the BM of normal controls (n = 8) and JAK2V617I-positive samples. (C) Numbers of BFU-E, CFU-GM, and CFU-GEMM in the BM of NC (n = 8) and JAK2V617I-positive (n = 4) cases. (D) Numbers and sizes of BM CFU-Mk in NC (n = 3) versus JAK2V617I (n = 4) patients. Error bars represent SEM. P values are shown if < .05.
JAK2V617I CD34+ cells efficiently engraft in a xenotransplantation assay
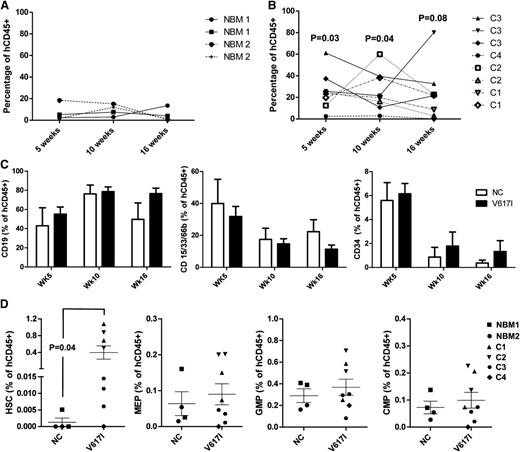
To determine whether JAK2V617I CD34+ cells were capable of sustaining long-term multilineage reconstitution, we transplanted CD34+ BM cells from JAK2V617I-positive cases into NSG mice with 2 NBM controls in parallel. Engraftment was monitored by serial BM aspiration followed by a final analysis at 18 weeks’ post transplant. Engraftment levels of human CD45+ cells were higher for JAK2V617I-positive cases than for NBM controls at 5 weeks (P = .03), 10 weeks (P = .04), and 16 weeks (P = .08; Figure 2A-B), although engraftment levels were variable and, in the case of C4, low. There was no significant difference in the relative proportion of B-lymphoid or myeloid cells as a proportion of human CD45+ cell engraftment, or in numbers of CD34+ cells (Figure 2C), although there was a suggestion that the latter were sustained at higher levels with time in JAK2V617I-positive cases. At 18 weeks after transplantation, a higher proportion of JAK2V617I-positive cases showed a presence of Lin-CD34+CD38–CD90+CD45RA– phenotypic HSCs (1/4 NCs versus 7/8 V617I-transplanted mice). Furthermore, numbers of phenotypic HSCs were also increased as a proportion of human CD45+ cells compared with NBM (P = .04; Figure 2D). Numbers of MEP, CMP, and GMP were not different (Figure 2D). Taken together with the increase in phenotypic stem cells in the PB and BM of JAK2V617I cases, these data support that germline JAK2V617I mutation might lead to an intrinsically mediated expansion of HSCs.
Long-term engraftment kinetics of germline JAK2V617I CD34+ cells after xenograft transplantation. Human CD45 engraftment kinetics for age-matched NBM cases: (A) controls, n = 2 subjects; 2 recipient mice per sample and (B) JAK2V617I, n = 4, cases C1-C4; 1-3 recipient mice per samples after injection of 100 000 CD34+ cells into NOD/LtSz-scidIL2Rgnull (NSG) mice. P values in (B) represent a comparison of JAK2V617I and NBM engraftment levels at each respective time. (C) Engraftment kinetics of CD19+ B cell, CD15/33/66b+ myeloid cells, and CD34+ progenitor cells expressed as a percentage of total hCD45+ cells. (D) Quantification of HSCs, MEPs, GMPs, and CMPs in the BM at 18 weeks after transplantation. Error bars represent SEM. P values are indicated if < .1.
Long-term engraftment kinetics of germline JAK2V617I CD34+ cells after xenograft transplantation. Human CD45 engraftment kinetics for age-matched NBM cases: (A) controls, n = 2 subjects; 2 recipient mice per sample and (B) JAK2V617I, n = 4, cases C1-C4; 1-3 recipient mice per samples after injection of 100 000 CD34+ cells into NOD/LtSz-scidIL2Rgnull (NSG) mice. P values in (B) represent a comparison of JAK2V617I and NBM engraftment levels at each respective time. (C) Engraftment kinetics of CD19+ B cell, CD15/33/66b+ myeloid cells, and CD34+ progenitor cells expressed as a percentage of total hCD45+ cells. (D) Quantification of HSCs, MEPs, GMPs, and CMPs in the BM at 18 weeks after transplantation. Error bars represent SEM. P values are indicated if < .1.
JAK2V617I induces cytokine hyperresponsiveness but significantly weaker constitutive activation than JAK2V617F
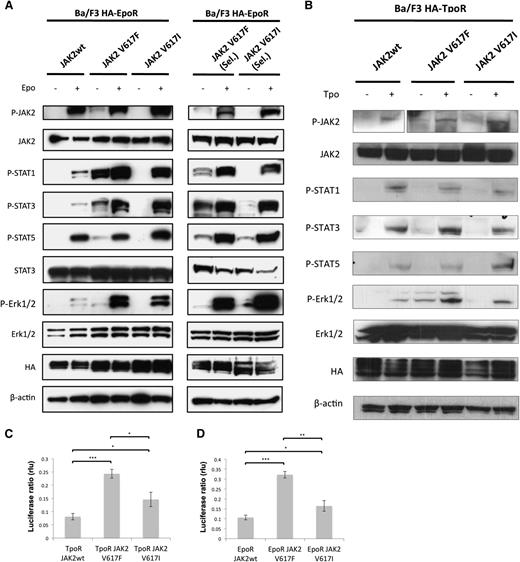
Finally, we explored whether the JAK2V617I mutation results in similar signaling consequences as the more common JAK2V617F mutation. Initial studies had revealed that JAK2V617I can lead to constitutive activation of JAK2 kinase domain.41 However, cytokine-independent colonies (CFU-GM, BFU-E, or CFU-Mk) were not observed in the PB or BM of JAK2V617I cases, in contrast to JAK2V617F-positive patients, in whom we observed cytokine-independent colonies in 6 of the 7 cases tested (data not shown). This suggests that the impact of JAK2V617I on signaling might be distinct from JAK2V617F. Here we obtained multiple additional lines of evidence demonstrating that signaling by JAK2V617I, although more active than wild-type JAK2, is weaker and possibly qualitatively different from that by JAK2V617F. First, in Ba/F3 EpoR–expressing cells, constitutive phosphorylation and activation were detected for JAK2, STAT1, STAT3, STAT5, and Erk1/2 in the case of JAK2V617F but were below the levels of detection for JAK2V617I cells (Figure 3A). Second, when mutant JAK2-expressing cells were allowed to become autonomous by selection in the absence of cytokines, JAK2V617I signaling was again much weaker than with JAK2V617F. For the latter, we detected strong levels of constitutive activation of STAT1, STAT3, and STAT5, whereas for autonomous Ba/F3 JAK2V617I cells, we detected weaker levels of constitutive STAT3 and STAT5 levels, and no activation of STAT1 (Figure 3A). In response to EPO stimulation, however, JAK2V617I resulted in markedly higher levels of downstream signaling compared with wild-type JAK2 and comparable with JAK2V617F for STAT1 as well as STAT3 and Erk1/2 in both autonomous and nonautonomous lines. STAT5 activation in response to EPO stimulation was similar to that seen for wild-type JAK2.
JAK2V617I induces only weak constitutive activation but marked cytokine hyperresponsiveness. (A) A representative analysis (1/2 independent experiments with similar results) of Ba/F3 EpoR cells that were engineered to overexpress equal levels of wild-type JAK2, JAK2V617F, or JAK2V617I by bicistronic retroviral transduction and cell sorting. Cells growing in medium supplemented with IL3 (left panel) or cells that acquired autonomous growth, namely cells expressing JAK2V617F or JAK2V617I (right panel, cells are denoted “Sel.”) were starved for 5 hours without serum and cytokines and then stimulated with EPO (20 U/mL) as indicated for 15 minutes, and then lysed in 1% NP40 buffer and assessed by Western blotting for specific phosphorylation at sites that reflect activation of JAK2, STAT1, STAT3, STAT5, and Erk1/2, and for total level of JAK2, STAT3, Erk1/2, HA, and β-actin protein expression. (B) A representative analysis (1/2 independent experiments with similar results) of Ba/F3 TpoR cells that were engineered to overexpress equal levels of wild-type JAK2, JAK2V617F, or JAK2V617I by bicistronic retroviral transduction and cell sorting. Cells growing in medium supplemented with IL3 were starved for 5 hours without serum and cytokines and then stimulated with Tpo (20 ng/mL) as indicated, and then lysed after 15 minutes in 1% NP40 buffer and assessed by Western blotting for specific phosphorylation at sites that reflect activation of JAK2, STAT1, STAT3, STAT5, and Erk1/2, and for total level of JAK2, STAT3, Erk1/2, HA, and β-actin protein expression. (C-D) Results of luciferase assays in γ2A fibrosarcoma JAK2-deficient cells transfected with cDNAs coding for TpoR (C) or EpoR (D) along with wild-type JAK2, JAK2V617F, or JAK2V617I, along with STAT5 and STAT-dependent firefly luciferase and pRLTK-driven renilla luciferase (rlu), for normalization. Dual luciferase was measured 24 hours after transfection. Results of 1 representative experiment of 3 independent experiments are shown. Data are expressed as means of triplicates, and error bars indicate SD. *P < .05, **P < .01, ***P < .001.
JAK2V617I induces only weak constitutive activation but marked cytokine hyperresponsiveness. (A) A representative analysis (1/2 independent experiments with similar results) of Ba/F3 EpoR cells that were engineered to overexpress equal levels of wild-type JAK2, JAK2V617F, or JAK2V617I by bicistronic retroviral transduction and cell sorting. Cells growing in medium supplemented with IL3 (left panel) or cells that acquired autonomous growth, namely cells expressing JAK2V617F or JAK2V617I (right panel, cells are denoted “Sel.”) were starved for 5 hours without serum and cytokines and then stimulated with EPO (20 U/mL) as indicated for 15 minutes, and then lysed in 1% NP40 buffer and assessed by Western blotting for specific phosphorylation at sites that reflect activation of JAK2, STAT1, STAT3, STAT5, and Erk1/2, and for total level of JAK2, STAT3, Erk1/2, HA, and β-actin protein expression. (B) A representative analysis (1/2 independent experiments with similar results) of Ba/F3 TpoR cells that were engineered to overexpress equal levels of wild-type JAK2, JAK2V617F, or JAK2V617I by bicistronic retroviral transduction and cell sorting. Cells growing in medium supplemented with IL3 were starved for 5 hours without serum and cytokines and then stimulated with Tpo (20 ng/mL) as indicated, and then lysed after 15 minutes in 1% NP40 buffer and assessed by Western blotting for specific phosphorylation at sites that reflect activation of JAK2, STAT1, STAT3, STAT5, and Erk1/2, and for total level of JAK2, STAT3, Erk1/2, HA, and β-actin protein expression. (C-D) Results of luciferase assays in γ2A fibrosarcoma JAK2-deficient cells transfected with cDNAs coding for TpoR (C) or EpoR (D) along with wild-type JAK2, JAK2V617F, or JAK2V617I, along with STAT5 and STAT-dependent firefly luciferase and pRLTK-driven renilla luciferase (rlu), for normalization. Dual luciferase was measured 24 hours after transfection. Results of 1 representative experiment of 3 independent experiments are shown. Data are expressed as means of triplicates, and error bars indicate SD. *P < .05, **P < .01, ***P < .001.
We also tested signaling in Ba/F3 TpoR cells. In this cell-line model, in the absence of selection for autonomous growth, JAK2V617F showed evidence of weak cytokine-independent activation for Y1007 phosphorylation in JAK2, STAT3, and Erk1/2 (Figure 3B). Similarly to Ba/F3 EpoR cells, JAK2V617I induced little cytokine-independent signaling. In response to Tpo stimulation, signaling was generally weaker than that seen with EPO stimulation. However, JAK2V617I-expressing cells notably showed strong pJAK2 and pSTAT5 activation with Tpo treatment. By dual-luciferase assays measuring STAT5-dependent transcription with the pGRR5 reporter, V617I did demonstrate constitutive signaling in the presence of EpoR or TpoR, higher than for wild-type JAK2, however consistently weaker than that of V617F (Figure 3C-D).
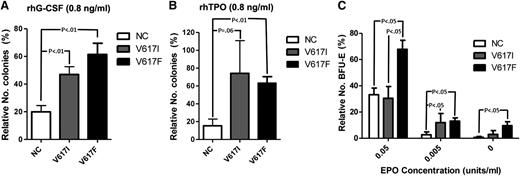
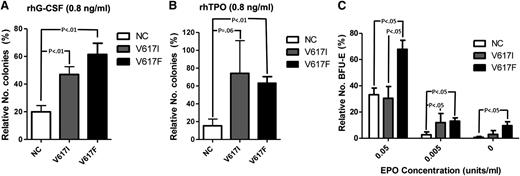
To explore this further in primary hematopoietic cells, we carried out cytokine response assays to assess colony formation under conditions of low levels of cytokine stimulation. Hematopoietic cells from JAK2V617I-positive cases showed increased colonies in response to low concentrations of G-CSF combined with SCF compared with JAK2 wild-type samples, but not significantly different from those with JAK2V617F-positive MPN samples (Figure 4A). Similarly, low levels of TPO combined with SCF induced more colonies for JAK2V617I than for wild-type JAK, although without reaching statistical significance (P = .06), and to a similar level as seen for JAK2V617F (Figure 4B). Notably, at EPO 0.05 U/mL, BFU-E formation was increased for JAK2V617F, but not JAK2V617I, cases when compared with wild-type JAK2 cases (Figure 4C). However, at an even lower concentration of EPO (0.005 U/mL), JAK2V617I cases also showed more BFU-Es than wild-type JAK2 cases (Figure 4C), although no patient with JAK2V617I showed evidence of polycythemia.25
Cytokine response assays. (A-B) Colony formation with rhSCF at 50 ng/mL and (A) rhG-CSF (Amgen) or (B) rhTPO, each at 100 ng/mL or 0.8 ng/mL. Results are from normal subjects (n = 6), JAK2V617I cases (n = 4), and JAK2V617F-positive MPNs (n = 6), expressed as numbers of colonies formed with 0.8 ng/mL rhG-CSF or rhTPO as a percentage of colony numbers formed with 100 ng/mL rhG-CSF or rhTPO. (C) The numbers of BFU-E generated with conditioned methylcellulose (see Methods) in the presence of rhEPO at 5 U/mL, 0.05 U/mL, or 0.005 U/mL, or in the absence of EPO. Results are expressed as a percentage relative to total numbers of BFU-Es generated at 5 U/mL for normal controls (n = 4), JAK2V617I cases (n = 4), and JAK2V717F cases (n = 7). P values are shown if < .1. Error bars represent SEM.
Cytokine response assays. (A-B) Colony formation with rhSCF at 50 ng/mL and (A) rhG-CSF (Amgen) or (B) rhTPO, each at 100 ng/mL or 0.8 ng/mL. Results are from normal subjects (n = 6), JAK2V617I cases (n = 4), and JAK2V617F-positive MPNs (n = 6), expressed as numbers of colonies formed with 0.8 ng/mL rhG-CSF or rhTPO as a percentage of colony numbers formed with 100 ng/mL rhG-CSF or rhTPO. (C) The numbers of BFU-E generated with conditioned methylcellulose (see Methods) in the presence of rhEPO at 5 U/mL, 0.05 U/mL, or 0.005 U/mL, or in the absence of EPO. Results are expressed as a percentage relative to total numbers of BFU-Es generated at 5 U/mL for normal controls (n = 4), JAK2V617I cases (n = 4), and JAK2V717F cases (n = 7). P values are shown if < .1. Error bars represent SEM.
These data demonstrate that compared with the JAK2V617F mutation, the JAK2V617I mutation results in only weak constitutive activation, as supported by lack of the cytokine-independent progenitor growth in JAK2V617I-positive cases that is characteristic of the JAK2V617F mutation. Rather, the JAK2V617I mutation results in a reduction in the threshold for cytokine-induced signaling and stem/progenitor cell proliferation.
Discussion
In the present studies, we carried out the first detailed characterization of the hematopoietic phenotype associated with germline JAK2 mutation to gain insight into the role of JAK2 mutations in MPN disease pathogenesis. We first set out to determine whether germline JAK2V617I mutation might be sufficient to induce the observed thrombocytosis phenotype, or whether collaborating MPN-associated mutations are required. As was reported previously, the high penetrance of the clinical phenotype,25 the relatively young age of disease manifestation, and the lack of clonality in the investigated female subjects as established here through XCIP clonality analysis, collectively point to JAK2V617I being the sole mutation causing the observed thrombocytosis. Moreover, we previously demonstrated absence of MPL and THPO gene mutations and lack of copy number abnormalities or areas of uniparental disomy in these cases.25 Using a combination of extended targeted gene sequencing and exome sequencing to exclude additional established chronic and blast-phase MPN-associated mutations, in the present studies we provide further genetic support toward the germline JAK2V617I mutation being sufficient to induce the observed thrombocytosis phenotype in the absence of other collaborating mutations. However, it is important to note that exome analysis cannot categorically exclude that other mutations may be present and involved in the thrombocytosis observed in germline JAK2V617I cases, because sequencing data are only definitive when a mutation is detected, and a negative result does not exclude that collaborating mutations might exist. Nevertheless, taken together with the homogenous and high-penetrance phenotype and lack of evidence for clonality associated with germline JAK2V617I, these data strongly support that JAK2V617I is likely to be the key driver mutation sufficient to induce the observed homogeneous and clinically manifest ET-like phenotype, with a mild/moderate thrombocytosis and no evidence of polycythemia, leucocytosis, or disease transformation to myelofibrosis or leukemia.25 Thus, the diverse phenotypes associated with somatic JAK2V617F mutation might, at least in part, relate to acquisition of other molecular events or genetic modifiers. Similarly, an inherited mutation of MPL causes hereditary thrombocytosis, but when arising as a somatic mutation, it is associated with both ET and myelofibrosis phenotypes.46,47
Thus, the findings presented here are consistent with a model of MPN pathogenesis in which JAK2 mutation may cause an MPN phenotype as a “single hit.” However, an obvious difference between germline and somatic JAK2 mutations is that the latter occurs in a single cell, which must then undergo clonal expansion because it has been estimated that JAK2V617F needs to be present in at least 1% to 2% of HSCs before a clinical MPN phenotype emerges.3 Indeed, the somatic JAK2 mutant allele burden in the primitive CD34+CD38– compartment in patients with ET ranges from 10% to 20% in most patients,18 consistent with 20% to 40% of HSCs carrying the mutation. This is in contrast to the reported finding of a cell-intrinsic suppression of HSCs in a murine model of human JAK2V617F-induced ET.19 Other mouse models of JAK2V617F have not shown a similar suppression of HSCs; however, they either express the murine form of the mutation or the human JAK2V617F using a bacterial artificial chromosome–transgenic approach, which may not faithfully recapitulate expression of JAK2 in the hematopoietic hierarchy.20,21,24 Thus, the impact of physiologically expressed JAK2 mutation on HSCs in humans remains uncertain. Moreover, the impact of JAK2 mutation on primitive human hematopoiesis in MPNs is unclear, with one study suggesting normal numbers of HSCs in patients with JAK2V617F PV and ET18 and another study showing an expansion of HSCs in these patients.17 In our investigation, studying individuals with germline-activating JAK2V617I mutation offered a unique opportunity to explore the impact of activating JAK2 mutation on human hematopoietic stem/progenitor cells in the likely absence of other collaborating genetic events. In the present study, we demonstrate an expansion of phenotypic HSCs in the PB of JAK2V617I-positive cases, and a particularly marked expansion of HSCs in the BM of the oldest patient studied (C1). At the very least, these data seem incompatible with a depletion of HSCs caused by JAK2V617I mutation and rather would suggest that HSCs may expand with time. Within the variability and limitations of the xenograft assay, further support that JAK2V617I might induce an intrinsically mediated expansion of HSCs was gained from the observed high-level long-term engraftment that was seen in xenotransplant assays with enhanced maintenance of HSCs when compared with normal controls. However, the small numbers of cases studied and variability of results necessitate a degree of caution in interpretation of these data in this regard. Furthermore, because the JAK2V617I mutation is present in all cells in patients with a germline mutation, these studies could not address whether JAK2V617I HSCs have a clonal advantage over wild-type JAK2-expressing cells, although our findings are compatible with this possibility. Notably, JAK2V617I patients also had increased CFU-GMs and, to a lesser degree, CFU-Mks, whereas, in keeping with the lack of polycythemia in patients with JAK2V617I mutation, BFU-Es were not increased. Thus, together with the lack of other MPN-associated mutations, these findings are compatible with a model in which JAK2 mutation as a “single hit” is sufficient to expand the circulating HSC pool and induce an MPN phenotype.
Interestingly, it has been proposed that germline JAK2V617F might be embryolethal in humans because of the severe phenotype observed in some mouse models.22 Although the germline JAK2V617I mutation affects the same amino acid as the much more common JAK2V617F mutation, it is possible that the 2 mutations differentially affect signaling, a possibility supported by several lines of experimental data in our studies. Initial studies had revealed that JAK2V617I can lead to constitutive activation of JAK2 kinase domain.41,48 Indeed, here we confirmed that overexpression of JAK2V617I in Ba/F3 EpoR cells results in autonomous growth for JAK2V617I-transduced cells, but not for wild-type JAK2,29 indicating some degree of constitutive activation. However, we also demonstrate that JAK2V617I exerts only weak constitutive signaling when compared with JAK2V617F. Rather, we provide evidence in support of the hematopoietic phenotype of JAK2V617I being the result of marked cytokine (EPO and TPO) hyperresponsiveness, considerably lowering the threshold for cytokine-induced activation, as supported by our previous observations.25 For EPO, this was particularly evident for STAT1 and STAT3 and less so for STAT5 compared with wild-type JAK2 signaling, a finding of particular relevance for the megakaryocytic rather than erythroid phenotype, because the balance of STAT1 and STAT5 signaling has been shown to correlate with ET phenotype.49 Moreover, although cytokine-independent activation of STATs was significantly weaker for JAK2V617I when compared with JAK2V617F, Erk activation was clearly not induced by JAK2V617I in the absence of ligand in sorted cells expressing either EpoR or TPOR. Thus, although JAK2V617F induces strong constitutive activation in addition to cytokine hyperresponsiveness, JAK2V617I primarily induces the latter. In keeping with this, cytokine-independent colonies were not seen with JAK2V617I, in contrast to JAK2V617F. Conversely, cytokine-response colony assays demonstrated that the presence of JAK2V617I results in hyperresponsiveness to TPO, as well as G-CSF, and also to some degree EPO, despite the lack of polycythemia in patients with JAK2V617I.25 These findings are of relevance both for the ET-like phenotype induced by JAK2V617I and also the HSC phenotype, because TPO is a key cytokine for HSC maintenance,50,51 and the “strength” of mutant JAK2 signaling may correlate with ET versus PV phenotype.23
Our finding of limited constitutive activation by JAK2V617I compared with JAKV617F helps to explain the specific thrombocytosis phenotype conferred by a germline JAK2V617I mutation and suggests that germline JAK2V617F mutation might be associated with a more distinct impact on HSCs and possibly a more severe MPN phenotype. In this regard, it is noteworthy that a recent preliminary report suggests that “canonical JAK2V617F” germline mutation occurred in one patient with a PV phenotype.28 Furthermore, a number of additional preliminary reports have detected other germline JAK2 mutations in patients with thrombocytosis.26,27 As additional germline JAK2 mutations are discovered, detailed characterization of the clinical, hematopoietic, and signaling phenotypes associated with specific mutations, as described in this study, will provide important and novel insights into the biology of distinct JAK2 mutations in MPN pathogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Céline Mouton for excellent technical assistance.
This work was supported by Leukaemia and Lymphoma Research (A.J.M., P.V., D.A.), a Career Development Program Fellowship from the Leukemia and Lymphoma Society (P.W.), Salus Sanguinis, Fondation Contre le Cancer, Fonds de la Recherche Scientifique-FNRS, Programs Pôles d’Attraction Interuniversitaires BCHM61B5, and Action de Recherche Concertées Action de Recherche Concertees of Université Catholique de Louvain, Belgium (C.P., A.D., S.N.C.), Medical Research Council (P.V., S.E.W.J.), and the Oxford Partnership Comprehensive Biomedical Research Centre (Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme (A.J.M., M.R., R.C., P.V., S.H., S.E.W.J., A.S.).
The views expressed in this article are those of the authors and not necessarily those of the Department of Health.
Authorship
Contribution: A.J.M. designed, performed and analysed experiments and wrote the manuscript; O.C., D.A., M.R., J.S., P.W., A.B., A.D., R.C., and C.P. performed and analysed experiments; R.E.G. designed and performed clonality analysis and analyzed experiments; S.M. and N.B. collected clinical data; S.H. designed, performed, and analyzed experiments; P.V. contributed to experimental design; N.C., S.N.C., and A.S. designed and analyzed experiments and helped write the manuscript; S.E.W.J. designed and analyzed experiments and edited the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adam Mead, Haematopoietic Stem Cell Biology Laboratory, Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Headington, Oxford, OX3 9DS UK; e-mail: adam.mead@imm.ox.ac.uk.
References
Author notes
S.N.C. and A.S. contributed equally to this study.