In this issue of Blood, Wechalekar and colleagues demonstrate the importance of treating all patients with amyloidosis, no matter how severe their situation is, because clinically important responses are seen.1 They also identify the adverse prognostic impact of a systolic blood pressure of <100 mm Hg.1
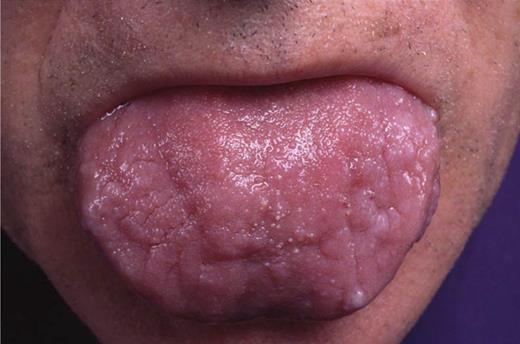
This article from the 4 largest amyloidosis centers in Europe is important because it reports on large numbers and reduces the referral biases that major medical centers see with these patients. Often, when patients present with advanced end-stage disease, they are not referred or do not survive to be seen in a center of expertise, and reported center outcomes are not reflective of an unselected population. Moreover, these patients are often excluded from clinical trials of therapy in amyloidosis. As a result, published phase 2 trials are not representative of the “real-world” situation with amyloidosis.2 All patients in this report were cardiac stage III3 defined by an amino-terminal fragment brain-type natriuretic peptide (NT-proBNP) of >332 ng/L and a cardiac troponin T of >0.035 µg/L or a cardiac troponin I >0.1 µg/L. These patients do poorly in large part due to late diagnosis and an inability to aggressively treat patients with poor performance status. Patients with cardiac amyloidosis are often diagnosed late because the condition is a classic example of heart failure with preserved systolic function and normal ejection fraction.4 Physical findings such as purpura and enlargement of the tongue (see figure) are useful when present but are generally absent in 85% of patients, so a high index of suspicion is required.
A number of staging systems exist to help prognosticate outcomes in amyloidosis, most recently, a combination of cardiac biomarkers and the difference between involved and uninvolved immunoglobulin free light chains, a measure of tumor mass.5 For patients with advanced cardiac failure, the severity of heart impairment eliminates the relevance of the free light chain from the statistical model. A systolic blood pressure of <100 mm Hg, reflecting advanced stiffening of the heart, poor diastolic filling, and a reduced stroke volume, resulting in a reduced systolic blood pressure, carries paramount importance in predicting outcome. Even with the poor prognosis in this group, the overall hematologic response rate on an intent-to-treat basis was 33% with 12% complete responses. It appears that no patient with amyloidosis should be denied a trial of therapy. The number of patients in each treatment category does not allow selection of any superior therapy: patients responded to combinations of melphalan and dexamethasone; combinations using thalidomide; and combinations using either bortezomib, lenalidomide, or both. A small subset of responders may become suitable candidates for safe stem cell transplantation6 with reported 10-year survivorship.7
Hematologists that see patients with monoclonal gammopathy of undetermined significance must be on the lookout for amyloidosis, which can manifest as simple fatigue or weight loss and be misattributed to multiple myeloma, simply because the bone marrow shows 10% plasma cells. Over 25 years of monitoring, one-quarter of patients with monoclonal gammopathy of undetermined significance will develop a serious plasma cell proliferative disorder. What is often overlooked is that one-eighth of these patients, or ∼3%, will develop light chain amyloidosis. This can occur without a rise in the size of the monoclonal protein, and the monitoring physician must be aware of the development of diarrhea, edema, or lower extremity paresthesias. Others have suggested that measurement of the NT-proBNP become part of the evaluation of patients with monoclonal gammopathy of undetermined significance who are being observed to detect early amyloid cardiomyopathy.
Amyloidosis is rare, and because no specific diagnostic test exists, lacking a high index of suspicion, it can easily be overlooked. The responses to chemotherapy reported in this manuscript clearly benefit our patient population even with advanced cardiac disease. The development of new agents for the treatment of amyloidosis include pomalidomide8 and the potential for carfilzomib, MLN-9708, and the exciting development of amyloid-specific monoclonal antibodies9 offers great hope for the future of our patients.
Conflict-of-interest disclosure: The author declares no competing financial interests.