Key Points
Activation-induced Notch signaling is crucial for both generation and effector functions of primary naive CD8 T cells.
Notch signaling is needed for expansion and IFNγ release but not for cytolytic activity of established effector CD8 T cells.
Abstract
The generation of effector CD8+ T cells with lytic capacity is crucial for tumor control. Dendritic cells (DCs) provide important signals to promote naive CD8+ T cell priming and activation of effector T cells. Here, we report that the Notch pathway has an important role in both these processes in human CD8+ T cells. Activated monocyte-derived DCs express Notch ligands Jagged1 and Delta-like4, whereas naive CD8+ T cells express Notch2. The role for Notch signaling in CD8+ T cell priming was determined using an ex-vivo model system in which tumor antigen–specific primary CD8+ T cell responses were measured. Inhibition of Notch using γ-secretase inhibitors or soluble Delta-like4-Fc during activation reduced expansion of antigen-specific CD8+ T cells, which was mirrored by decreased frequencies of interferon (IFN)γ-, tumor necrosis factor-α–, and granzymeB-producing CD8+ T cells. Moreover, T cells primed when Notch signaling was prevented are functionally low-avidity T cells. In addition, Notch partially regulates established effector T cell function. Activation-induced Notch signaling is needed for IFNγ release but not for cytolytic activity. These data indicate that Notch signaling controls human CD8+ T cell priming and also influences effector T cell functions. This may provide important information for designing new immunotherapies for treatment of cancer.
Introduction
Activated CD8+ T cells are essential for successful elimination of invading pathogens and transformed cells. A critical feature of these activated T cells is their ability to recognize specific peptide-MHC (pMHC) complexes with sufficient avidity to induce lytic activity and expression of effector cytokines, such as interferon (IFN)γ and tumor necrosis factor (TNF)α.
The efficacy of CD8+ T cell priming critically depends on interaction of the T cell receptor (TCR) with pMHC complexes, as well as on signals derived after engaging costimulatory receptors. It has become clear that members of both the Ig and TNF receptor family, such as CD28, CD27, and OX40, provide essential signals for activation, proliferation, survival, and differentiation of naive CD8+ T cells.1-4 Recent data indicate that the Notch pathway also modulates activation and differentiation of peripheral T cells.5
In mammals, 4 Notch homologs (Notch1-4) and 5 ligands of the Delta-like families (DLL1; 3 and 4) and Jagged families (Jag1 and Jag2) have been described.6 These transmembrane receptors and ligands are typically presented on opposing cells, implying that ligand binding is involved in cell-cell communication. Indeed, Notch is a highly conserved pathway in development.6 Ligation of Notch receptor by its ligand induces proteolytic processing, including a final cleavage by a γ-secretase, resulting in the release of the Notch intracellular domain (NICD). The liberation of NICD from the membrane allows it to translocate to the nucleus, where it participates in a transcriptional activator complex with CSL (CBF1/RBPJ-κ in vertebrates, Su(H) [Suppressor of hairless] in Drosophila, Lag1 in Caenorhabditis elegans) and Mastermind to activate the expression of target genes. Target genes include the transcription factors Hes1, Hes5, NF-κB, and NF-AT.7
Notch receptors and their ligands are expressed by T cells and antigen-presenting cells (APCs), respectively. Most knowledge on Notch signaling in T cells comes from studies on T cell development in the thymus using transgenic and knockout mice.8 Recent studies also implicated the Notch pathway in peripheral T cell responses. For instance, Notch signaling is required for the activation of peripheral CD4 T cells in mice.9-12 Moreover, Notch ligands expressed on dendritic cells (DCs) have been shown to instruct T helper cells to commit to the Th1, Th2, Th17, or Treg lineage.12-17
The proposed involvement of Notch in activation and differentiation of CD8+ T cells is less clear. TCR signaling in murine CD8+ T cells has been shown to activate Notch signaling that contributes to the development of effector cytotoxic T lymphocytes (CTL).18 Murine CD8+ T cells activated in the presence of either a pharmacological inhibitor of Notch signaling (γ-secretase inhibitor) or an anti-Notch1 antibody produced less IFNγ, perforin, and granzyme B, herewith reducing lytic activity.18 The need for Notch signaling to obtain effector status was also shown in a study using murine DCs overexpressing the Notch ligand DLL1.19 By contrast, a tolerizing role for Notch signaling in CD8+ T cells has been advocated.20 These differences in outcome may be because of the use of different cell types for gain-of-function studies and different conditions in which the role of the particular ligand is investigated.
Although data are contradictory, it remains evident that Notch signaling can alter the differentiation potential of CD8+ T cells, at least in a murine setting. However, no data are available on the role of physiological Notch-Notch ligand interactions in the activation and effector function of human CD8+ T cells. We therefore set out to analyze the expression of Notch pathway components in human naive and effector T cells on activation and determine how Notch signaling influences activation of tumor-specific CD8+ T cells.
Methods
Antibodies and reagents
Antibodies specific for human Notch1 (clone MHN1-519), granzyme B (clone 16G6), and CD8 AF488 and PE (clones OKT-8 and SK1) were purchased from eBiosciences (San Diego, CA); Notch2-biotin (clone MHN2-25) was purchased from Miltenyi (Auburn, CA). Anti-NICD antibody was obtained from Abcam (Cambridge, MA). Antibodies specific for IFNγ (clone 25723.11), TNFα (clone 6401.1111), CD107a (clone H4A3), CD8 APC (clone SK1), and streptavidin-APC were obtained from BD Pharmingen (San Diego, CA). The γ-secretase inhibitors compound E {(S,S)-2-[2-(3,5-difluorophenyl)-acetylamino]-N-(1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-propionamide} and GSI X ({1S-benzyl-4R-[1-(1S-carbamoyl-2-phenethylcarbamoyl)-1S-3-methylbutylcarbamoyl]-2R-hydroxy-5-phenylpentyl}carbamic acid tert-butyl ester) were obtained from Calbiochem (Darmstadt, Germany) and used at the indicated concentrations. αCD3/CD28 Dynabeads were purchased from Invitrogen (Oslo, Norway) and used at 1 × 106 beads/3 × 106 cells. Human DLL4-Fc was used at 5 µg/mL (Enzo Life Sciences, Farmingdale, NY).
Peptide synthesis and generation of HLA-A2-peptide tetramers
Peptides were synthesized using Fmoc amino acids and PyBop/NMM chemistry. Synthetic peptides were analyzed by reversed phase high-performance liquid chromotography (purity was ≥85%) and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (expected masses were confirmed). Tetrameric HLA-A2-peptide complexes were prepared as previously described.21
Generation and activation of monocyte-derived DCs
Monocytes were isolated from HLA-A2+ peripheral blood mononuclear cells from healthy donors using CD14-MACS beads (Miltenyi, Germany). All blood donors gave informed consent. Monocyte-derived DCs (moDCs) were generated by culturing monocytes for 6 days in RPMI 1640 medium (Invitrogen) containing 10% fetal calf serum, 50 U/mL penicillin, and 50 μg/mL streptomycin in the presence of recombinant human IL-4 and granulocyte macrophage–colony-stimulating factor (500 and 800 U/mL respectively; Biosource, Camarillo, CA). On day 6, DCs were harvested and activated by incubation with either 100 ng/mL lipopolysaccharide (LPS) or by coculture with CD40L-expressing mouse fibroblastic L cells22 for the indicated time.
Activation of Mart-1–specific CD8+ T cells by moDCs
A melanoma–associated antigen MelanA (Mart-1)26-35–specific CD8+ T cell clone was generated and cultured as described previously.23 Activated moDCs (104) were seeded in 96-well U-bottom plates and pulsed with 3 µg/mL Mart-126-35 (27L) or an HLA-A2 binding control peptide (CMVpp65495-503; 3 µg/mL) for 2 hours at 37°C. After washing, 5 × 104 Mart-1–specific T cells were added in the presence of 1 µg/mL Brefeldin A, and 24 hours later, intracellular levels of IFNγ or TNFα were analyzed by flow cytometry (Calibur; Becton Dickinson).
Antigen presentation to primary CD8+ T cells in an autologous setting
Human HLA-A2.1+–activated moDCs (50 000/well) were incubated with Mart-126-35(27L) peptide (3 µg/mL) in a flat-bottom 48-well plate for 2 hours. After extensive washing, autologous naive CD45RAhiCD27hi CD8+ T cells, isolated from peripheral blood using a magnetic-activated cell sorting untouched naive CD8+ T cell isolation kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), were added (106/well) in the presence of 10 ng/mL IL-7 (Peprotech, Hamburg, Germany). Three days later, fresh Iscove modified Dulbecco medium containing 10 ng/mL IL-7 and 50 IU/mL IL-2 (Proleukin) was added. At day 7, cells were harvested, counted, and stained with Mart-1-tetramer-APCs and analyzed by flow cytometry (Calibur; Becton Dickinson). Alternatively, intracellular levels of effector cytokines were analyzed. Hereto, cells were restimulated with indicated concentrations of Mart-126-35(27L) in the presence of 1 μg/mL Brefeldin A (Sigma) for 5 hours, and cytokine expression was measured by intracellular staining and flow cytometry.
Analysis of CD107a expression
Expression of CD107a on CD8+ T cells was examined as described by Betts et al.24 In brief, cells were mixed and incubated with Golgistop, anti-CD49d, anti-CD28, and phycoerythrin-labeled anti-CD107a or isotype control antibody (mouse IgG1; BD) in the presence or absence of Mart-1 peptide (3 μg/mL) for 5 hours. As a positive control, phorbol 12-myristate 13-acetate (PMA) and ionomycin stimulation was used at 10 and 500 ng/mL, respectively. After incubation, cells were washed and stained with anti-CD8 at 4°C, resuspended in phosphate-buffered saline containing 1% bovine serum albumin, and analyzed by flow cytometry.
CFSE-based cytotoxicity assay
To examine the influence of Notch signaling on the cytotoxic potential of effector T cells, we used a flow-based in vitro cytotoxicity assay with modifications. JY Epstein-Barr virus-transformed lymphoblastoid cell line (EBV-LCL) was labeled with either 3 or 0.3 µM carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen; CFSEhi and CFSElo cells, respectively) for 10 minutes at 37°C. CFSEhi cells were pulsed with 3 μg/mL Mart-1 peptide for 60 minutes at 37°C. CFSElo cells were pulsed with the same amount of a control peptide. Both cell populations were washed and combined at equal ratios, and 1 × 104 cells of each population were plated at 100 μL/well in 96-well microtiter plates. CTLs were added at different effector-target ratios. Plates were incubated at 37°C overnight. Thereafter, anti-CD8 antibodies were added to distinguish the effector cells and incubated at 4°C for 15 minutes. Wells were harvested and directly analyzed by flow cytometry. The specific cytolytic activity was calculated using the following equation: 1 – [(CFSEhi/CFSElo in wells containing Mart-1-specific T-cells)/(CFSEhi/CFSElo in control wells)] × 100.
Annexin V/7AAD assay for apoptosis
The Mart-1–specific CD8+ T cell clone was seeded at 1 × 106/mL in Iscove modified Dulbecco medium containing 50 U/mL penicillin, 50 μg/mL streptomycin, and 10% human serum. GSI or dimethylsulfoxide control was added, and at the indicated time periods, cells were stained with Annexin V fluorescein isothiocyanate (BD Pharmingen) and 7-aminoactomycin D (Invitrogen) and analyzed by flow cytometry.
Quantitative real-time reverse transcription-PCR
Total RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA) combined with a DNase treatment to remove contaminating DNA (Qiagen). cDNA was synthesized using the Reverse Transcription System kit (Promega) following the manufacturer’s guidelines. Real-time polymerase chain reaction (PCR) reactions were performed using the SYBR Green method in an ABI 7900HT sequence detection system (Applied Biosystems), with GAPDH as internal control. Samples were analyzed in triplicate and normalized to GAPDH. Primers were obtained from Invitrogen, and the sequences were as follows: GAPDH forward: 5′ CCT GTT CGA CAG TCA GCC G-3′, GAPDH reverse: 5′-CGA CCA AAT CCG TTG ACT CC-3′; Notch1 forward: 5′-ATC CTG ATC CGG AAC CGA G-3′; Notch1 reverse: 5′-CGT CGT GCC ATC ATG CAT-3′; Notch2 forward: 5′- GCA GTG CTG GAA GCT TGA GTA G-3′; Notch2 reverse: 5′-CCA GGA CCA TAC CAA ACA TCT CAT-3′; Hes1 forward: 5′-TGG AAA TGA CAG TGA AGC ACC T-3′; Hes1 reverse: 5′- GTT CAT GCA CTC GCT GAA GC-3′; Jagged-1 forward: 5′-GCG TTG CCC ACT TTG AGT AT-3′, Jagged-1 reverse: 5′-TTT TGT TGC CAT TCT GCT CA-3′; Jagged-2 forward: 5′-CGA GCG AGT GTC GCA TGC CGG-3′, Jagged-2 reverse: 5′-TGT TGC CGT ACT GGT CGC AGG-3′; Delta-like 1 forward: 5′-GGC ACC TTC TCT CTG ATT ATT G-3′, Delta-like 1 reverse: 5′-CGG CTG ATG AGT CTT TCT GG-3′; Delta-like 3 forward: 5′-GAG ACA CCC AGG TCC TTT GA-3′, Delta-like 3 reverse: 5′-CAG TGG CAG ATG TAG GCA GA-3′; Delta-like 4 forward: 5′-GCG AGA AGA AAG TGG ACA GG-3′, Delta-like 4 reverse: 5′-ACA GTA GCT GCC CGT CAA TC-3′.
Statistical analysis
Results of experiments were analyzed either using a one-way ANOVA, followed by a Bonferroni posttest or unpaired Student t test. Values were considered to be significantly different when P < .05.
Results
Human CD8+ T cell priming depends on Notch signaling
The first goal of our research was to specify the role of Notch signaling in human CD8+ T cell priming. Hereto, we set up an ex vivo model system taking advantage of the high frequency of naive CD8+ T cells specific for an epitope derived from the human Mart-1 in peripheral blood of HLA-A2+ healthy individuals.25,26 Naive HLA-A2+ CD8+ T cells could be physiologically activated by autologous DCs loaded with the Mart-126-35(27L) peptide. First, the presence of Notch ligands on the DCs and of Notch receptors on the CD8+ T cells was assessed. DCs were stimulated with LPS for up to 6 hours, and expression of all 5 Notch ligands was determined by quantitative reverse transcription-PCR (qRT-PCR) (Figure 1A). Transcripts of the ligands DLL4 and Jag1 were present in high amounts on stimulation. By contrast, DLL1, DLL3, and Jag2 mRNA expression was either very low or undetectable. DCs that were activated using αCD40 or poly I:C rendered comparable expression profiles (data not shown). Expression of Notch receptors was determined on resting and αCD3/αCD28-stimulated CD8+ T cells from peripheral blood using specific antibodies and flow cytometry. Notch2 was already present on resting CD8+ T cells, whereas Notch1 expression was very low at baseline (Figure 1B). On αCD3/αCD28 activation, expression of both receptors was up-regulated compared with the nonactivated CD8+ T cells.
Components of the Notch pathway are present on human moDCs and CD8+ T cells. (A) Relative expression of the five Notch ligands was determined by qRT-PCR in moDCs stimulated with LPS (100 ng/mL) for the indicated times. Samples were analyzed in triplicate. Shown is a representative example of 10 different donors. (B) Membrane expression of Notch1 and Notch2 was determined on CD8+ T cells by flow cytometry. PBLs were cultured with or without αCD3/CD28 microbeads for 4 days in the presence of IL-2 (30 IU/mL). The histograms shown represent live CD8+ T cells. Solid histogram, isotype control; black line, resting CD8+ T cells; gray line, activated CD8+ T cells.
Components of the Notch pathway are present on human moDCs and CD8+ T cells. (A) Relative expression of the five Notch ligands was determined by qRT-PCR in moDCs stimulated with LPS (100 ng/mL) for the indicated times. Samples were analyzed in triplicate. Shown is a representative example of 10 different donors. (B) Membrane expression of Notch1 and Notch2 was determined on CD8+ T cells by flow cytometry. PBLs were cultured with or without αCD3/CD28 microbeads for 4 days in the presence of IL-2 (30 IU/mL). The histograms shown represent live CD8+ T cells. Solid histogram, isotype control; black line, resting CD8+ T cells; gray line, activated CD8+ T cells.
Next, we primed CD8+ T cells by loading DCs with Mart-1 peptide and cocultured with naive CD8+ T cells. To assess a role for Notch, we performed our experiments in the presence or absence of a GSI or vehicle control. Already at day 7 after initial stimulation, outgrowth of Mart-1–specific CD8+ T cells could be observed in medium and vehicle control cultures (Figure 2A; supplemental Figure 1 on the Blood website). However, cultures treated with GSI showed reduced outgrowth of Mart-1–reactive cells as illustrated by the lower percentage of HLA-A2/Mart-1 tetramer-positive CD8+ T cells compared with vehicle-treated or control cultures (Figure 2A). GSI treatment of the cells did not affect cell viability (supplemental Figure 2). No positive staining was observed using an HLA-A2/control tetramer (Figure 2A, lower panels). Similar findings were obtained when interfering with Notch signaling by adding soluble Notch ligands.27 In these cultures, binding of endogenous ligands expressed by DCs was blocked by the added soluble DLL4-Fc, which also resulted in lower frequencies of HLA-A2/Mart-1-tetramer binding of CD8+ T cells compared with control cultures containing IgG1 (Figure 2B). Together, these experiments suggest that Notch signaling is required for activation and proliferation of naive human CD8+ T cells.
Notch signaling is important for human CD8+ T cell priming. Primary naive CD8+ T cells were cocultured with autologous Mart-1 peptide–loaded moDCs (3 µg/mL peptide), both isolated from an HLA-A2–typed healthy donor, as described in the Methods. Inhibition of Notch during CD8+ T-cell priming, either via (A) GSI (10 μM) or (B) soluble DLL4-Fc (5 μg/mL) resulted in diminished expansion of Mart-1–specific CD8+ T cells, as reflected by A2/Mart-1-tetramer staining using flow cytometry. The results shown are from 2 different donors. Similar results were obtained in 5 independent experiments.
Notch signaling is important for human CD8+ T cell priming. Primary naive CD8+ T cells were cocultured with autologous Mart-1 peptide–loaded moDCs (3 µg/mL peptide), both isolated from an HLA-A2–typed healthy donor, as described in the Methods. Inhibition of Notch during CD8+ T-cell priming, either via (A) GSI (10 μM) or (B) soluble DLL4-Fc (5 μg/mL) resulted in diminished expansion of Mart-1–specific CD8+ T cells, as reflected by A2/Mart-1-tetramer staining using flow cytometry. The results shown are from 2 different donors. Similar results were obtained in 5 independent experiments.
Next, we examined whether inhibition of Notch also impeded the differentiation of the expanded CD8+ T cells into functional effector cells. Hereto, the production of effector cytokines IFNγ and TNFα was measured by intracellular staining. On antigen-specific activation, IFNγ- and TNFα-producing Mart-1–specific T cells were detectable in control cultures (Figure 3A). A 60% reduction in the frequency of effector cytokine-producing T cells was observed in cultures in which Notch signaling was perturbed either using GSI or soluble DLL4-Fc (Figure 3A-B), corroborating the data on tetramer staining. No cytokine production was observed when DCs were loaded with a control HLA-A2 binding peptide, demonstrating the antigen specificity of the cells. Moreover, the amount of IFNγ+ cells was directly related to the strength of the stimulus, because lower peptide doses induced correspondingly lower amounts of IFNγ staining. In all doses tested, GSI treatment induced lower numbers of IFNγ+ cells compared with vehicle controls (Figure 3C, upper panel). Moreover, Mart-1–specific T cells expanded under GSI conditions may be of lower functional avidity as suggested by the reduced cytokine production on a per cell basis (Figure 3C, lower panel). These data imply that CD8+ T cells that differentiated under conditions with hampered Notch signaling have a reduced lytic capacity. Indeed, T cells primed in the presence of GSI have less cytotoxic activity, as indicated by the lower production of granzyme B (Figure 3D) and reduced mobilization of the degranulation marker CD107a to the cell surface (Figure 3E).
Inhibition of Notch signaling impairs acquisition of effector function by human CD8+ T cells. Human naive CD8+ T cells were cultured as described for Figure 2 in the presence or absence of (A) GSI (10 μM), (B) soluble DLL4-Fc (5 μg/mL), or the respective controls. Inhibition of Notch signaling during T cell priming reduced the amounts of IFNγ- and TNFα-producing CD8+ T cells. N = 5. (C) The inhibitory effect of GSI was independent of the strength of the stimulus; titration of the Mart-1 peptide to a lower dosage did not alter the inhibitory effect. As a measure for cytotoxic capacity, (D) intracellular levels of Granzyme B or (E) cell surface expression of CD107a was determined. Inhibition of Notch signaling reduced the expression of both cytolytic markers. Data are representative of 2 independent experiments.
Inhibition of Notch signaling impairs acquisition of effector function by human CD8+ T cells. Human naive CD8+ T cells were cultured as described for Figure 2 in the presence or absence of (A) GSI (10 μM), (B) soluble DLL4-Fc (5 μg/mL), or the respective controls. Inhibition of Notch signaling during T cell priming reduced the amounts of IFNγ- and TNFα-producing CD8+ T cells. N = 5. (C) The inhibitory effect of GSI was independent of the strength of the stimulus; titration of the Mart-1 peptide to a lower dosage did not alter the inhibitory effect. As a measure for cytotoxic capacity, (D) intracellular levels of Granzyme B or (E) cell surface expression of CD107a was determined. Inhibition of Notch signaling reduced the expression of both cytolytic markers. Data are representative of 2 independent experiments.
Inhibition of Notch controls effector T cell function
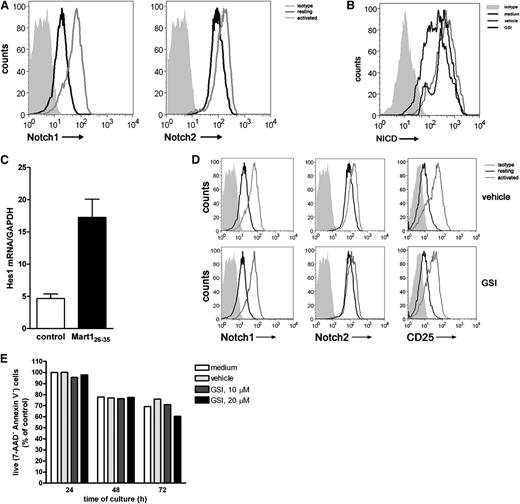
Ascertaining a clear role for Notch signaling in CD8+ T cell priming, we next assessed whether Notch is also required during the reactivation of established effector T cells. Hereto, a Mart-1–specific CD8+ T cell clone was used. Resting effector T cells express both Notch1 and Notch2 (Figure 4A), which is different from receptor expression on naive CD8+ T cells, which only express Notch2. Activation with antigen-pulsed DCs increased the expression of Notch1 and Notch2, which was confirmed by qRT-PCR (supplemental Figure 3). In addition, activation of the T cells resulted in accumulation of intracellular NICD, a process directly dependent on enzymatic activity of the γ-secretase complex (Figure 4B) and transcription of the downstream target Hes1 (Figure 4C). In the presence of GSI, accumulation of NICD was abrogated, resulting in diminished intracellular NICD levels (Figure 4B), confirming active inhibition of the Notch pathway by GSI. GSI treatment of the cells did not affect activation-induced surface expression of Notch1, Notch2, and CD25 (Figure 4D) or cell viability (Figure 4E).
Notch signaling is induced in established effector CD8+ T cells. A Mart-1–specific CD8+ T cell clone was incubated with nonpulsed (medium control) or Mart-1 peptide–pulsed moDCs, and 24 hours later, (A) surface expression of Notch1 and Notch2 and (B) intracellular expression levels of NICD1 were measured by flow cytometry and (C) Hes1 mRNA expression by qRT-PCR. Data are representative of 3 independent experiments. GSI treatment during activation prevented accumulation of (B) NICD intracellular but did not affect (D) surface expression of Notch1, Notch2, and CD25 nor did it affect cell viability, as measured by (E) annexin V/7AAD staining. Shown is a representative example of 3 independent experiments.
Notch signaling is induced in established effector CD8+ T cells. A Mart-1–specific CD8+ T cell clone was incubated with nonpulsed (medium control) or Mart-1 peptide–pulsed moDCs, and 24 hours later, (A) surface expression of Notch1 and Notch2 and (B) intracellular expression levels of NICD1 were measured by flow cytometry and (C) Hes1 mRNA expression by qRT-PCR. Data are representative of 3 independent experiments. GSI treatment during activation prevented accumulation of (B) NICD intracellular but did not affect (D) surface expression of Notch1, Notch2, and CD25 nor did it affect cell viability, as measured by (E) annexin V/7AAD staining. Shown is a representative example of 3 independent experiments.
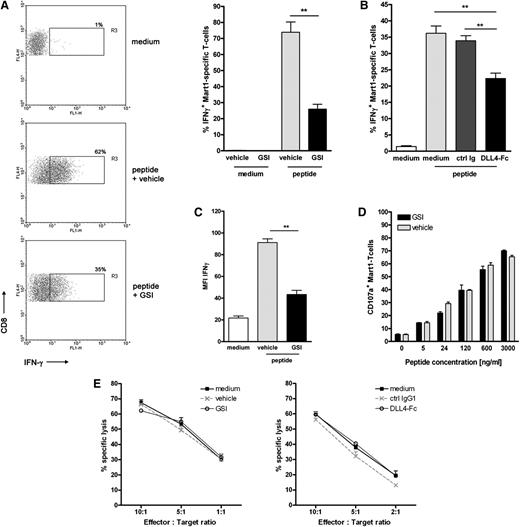
Similar to the primary naive CD8+ T cells, DC-mediated antigen-specific activation of the T cell clone led to a strong increase in IFNγ production (Figure 5A). Inhibition of Notch signaling using different sources of GSI significantly reduced the number of IFNγ-secreting T cells (Figure 5A; supplemental Figure 4). Similar data were obtained using soluble DLL4-Fc (Figure 5B). In addition, we observed that when Notch signaling was hampered, IFNγ production was also significantly decreased on a per cell basis (Figure 5C). By contrast, inhibition of Notch signaling did not affect the lytic function of the effector T cells. We measured equal cell surface mobilization of CD107a on Mart-1–specific CD8+ T cells on antigen-specific activation, irrespective of the presence of GSI or vehicle control (Figure 5D). Similarly, lysis of peptide-pulsed target cells by the Mart-1–specific effector T cells was not affected by addition of GSI or DLL4-Fc (Figure 5E) or when target cells were pulsed with a lower antigen dose (data not shown).
Notch signaling partially affects the effector function of established effector CD8+ T cells. Mart-1–specific CD8+ T cell clone was activated as described for Figure 4 in the presence or absence of Notch signaling inhibitors (A,C-E: 10 μM GSI or vehicle control; B: DLL4-Fc or 5 μg/mL control IgG). (A,B) The frequency of cells secreting IFNγ and (C) the amount produced per cell was determined 24 hours later using intracellular cytokine staining and flow cytometry (n = 5 independent experiments). As a measure for cytotoxic capacity, (D) cell surface expression of CD107a and (E) lytic capacity of the T-cell clone in a flow-based cytotoxicity assay were determined. Shown are representative examples of 3 independent experiments.
Notch signaling partially affects the effector function of established effector CD8+ T cells. Mart-1–specific CD8+ T cell clone was activated as described for Figure 4 in the presence or absence of Notch signaling inhibitors (A,C-E: 10 μM GSI or vehicle control; B: DLL4-Fc or 5 μg/mL control IgG). (A,B) The frequency of cells secreting IFNγ and (C) the amount produced per cell was determined 24 hours later using intracellular cytokine staining and flow cytometry (n = 5 independent experiments). As a measure for cytotoxic capacity, (D) cell surface expression of CD107a and (E) lytic capacity of the T-cell clone in a flow-based cytotoxicity assay were determined. Shown are representative examples of 3 independent experiments.
Discussion
This study provides the first evidence that the Notch pathway controls priming of human CD8+ T cells and activation of established effector T cells. Using either a γ-secretase inhibitor or by blocking Notch receptors via soluble DLL4 to prevent Notch signaling, we established that this pathway contributes to expansion of naive Mart-1–specific CD8+ T cells into effector cytokine–producing lytic CTLs. Moreover, established effector cells require Notch signaling during reactivation as blocking Notch signaling strongly diminished their function.
Exploiting the presence of high frequencies of naive Mart-1–specific CD8+ T cells in the peripheral blood of HLA-A2+ healthy donors, we were able to show that both expansion and differentiation of naive CD8+ T cells into effector T cells requires Notch signaling. Inhibition of this pathway using either GSI or soluble DLL4 prevented outgrowth of Mart-1–specific T cells, reflected by reduced frequencies of Mart-1/A2-tetramer staining of CD8+ T cells, as well as the number of IFNγ- and TNFα-producing T cells on antigen-specific restimulation.18,19 In comparison with GSI, addition of soluble DLL4 to inhibit Notch signaling had a less robust effect on T cell priming. Although GSIs are very robust compounds in inhibiting the activity of γ-secretase complexes, Notch receptors may have a lower affinity for soluble recombinant ligands than endogenous ligands, which are on a scaffold. In addition, during the DC-T cell coculture, Notch receptor expression and/or glycosylation changes, which may be an extra hurdle to blocking Notch signaling using soluble ligands, compared with GSI.28
Our data mirror previous data using murine T cells showing that Notch-mediated signaling was essential for activation of peripheral CD4+ and CD8+ T cells.9-11 However, in these studies, T cells were activated using an APC-free system or APCs that had been transduced with genes expressing a Notch ligand, whereas we reveal the influence of physiological levels of Notch ligands on human CD8+ T cell activation.12,19,29,30 In our experimental setup, we aimed to create an immunogenic environment by activating the moDCs with proinflammatory stimuli such as TLR ligands or aCD40. These stimuli induced the expression of DLL4 and Jag1 on human moDCs, confirming previous studies using human or murine DCs.12,31 Whether these ligands on moDCs exert different effects during CD8+ T cell priming needs further exploration.
The reduced outgrowth of CD8+ T cells reactive against Mart-1 when impairing Notch signaling may be caused by reduced proliferation and/or survival. In human peripheral CD4+ T cells, Notch signaling promotes cell cycle progression and proliferation.32 Notch signaling was shown to support G1-S progression of cell cycle via NICD-mediated binding and activation of the promoter of the G1 proteins cyclin D3, CDK4, and CDK6. Moreover, this was shown to be enhanced by NF-κB, a direct target of Notch signaling.11,32 The p50 subunit of NF-κB associates with the cyclin D3 promoter as well, thereby enhancing the Notch1-dependent promoter activity. In addition, murine naive CD4+ T cells activated in the presence of cross-linked recombinant DLL4 were very recently shown to be protected from apoptosis during expansion.33
We also demonstrated that acquisition of effector functions by the developing CTLs was constrained when Notch signaling was prevented. These data corroborate previous studies in mice.18,19,34 Notch1 can directly regulate IFNγ production as NICD interacts with NF-κB p50 on the IFNγ promoter and controls full cytotoxic activity of CTLs.11 NICD has been shown to form a stable complex with p300 and the transcription factor CREB1 on the promoters of the granzyme B and perforin genes.18,19 The clear effect of Notch2 on CD8+ T cells on controlling tumor growth and mediating in vivo cytotoxicity in the latter study may be explained by the reduced outgrowth of effector T cells, similar to our observations assessing the priming of Mart-1–specific CD8+ T cells.
In our experiments, we observed a reduced outgrowth of both Mart-1–specific naive CD8+ T cells, as well as Mart-1–specific established effector CD8+ T cells when Notch signaling was inhibited. We also found a strong increase in Hes1 expression in the established effector CD8+ T cells on peptide stimulation. It is therefore tempting to speculate that Hes1, the most commonly described Notch target gene, mediates the Notch-dependent effect. However, we could not detect an up-regulation of 2 other Notch target genes: Hes5 and Hey1 (data not shown). We can, therefore, not exclude a role for the noncanonical Notch signaling pathway, which functions by posttranslationally targeting the Wnt/β-catenin signaling pathway.
For priming into effector cells, CD8+ T cells require pertinent signals from DCs. Besides the triggering of TCR by pMHC complexes, binding of CD80/CD86 to CD28 is essential for activation and avoiding induction of tolerance.35 Our study underscores that codelivery of Notch signals exerts a profound effect on the outcome of antigen stimulation. This suggests that interplay between the TCR and Notch pathways regulates the outcome of the T cell response. The presence of naive Mart-1–specific T cells in the peripheral blood of HLA-A2+ donors allowed us to compare the contribution of Notch-mediated signaling to the activation and function of naive CD8+ T cells with that of an established effector T cell using the same antigen epitope and TCR triggering. Notch-mediated signaling proved to be required for the activation and subsequent production of effector cytokines by both naive and effector T cells. However, we observed that Notch differently affects the acquisition of lytic capacities in naive and effector T cells. Naive CD8+ T cells activated in the presence of Notch signaling inhibitors showed reduced lytic potential as inferred from a diminished frequency of CD107a expressing T cells and reduced expression of CD107a and GrB. These data corroborate murine studies showing that activation of murine CD8+ T cells that lack Notch2 possess reduced cytotoxic capacity and are less well capable of lysing tumor cells.19 Preliminary experiments suggest that, depending on the antigen and TCR affinity, T cells may differ in their reliance on Notch signaling for proliferation and/or differentiation.
Notch does not seem to affect this lytic function in established effector CTLs, as we demonstrate that they rapidly expressed CD107a and effectively lyse target cells, irrespective of the presence or absence of Notch signaling. This difference in effect of Notch signaling on activation of naive and effector T cells may lie in the distinct expression profiles of Notch receptors on these cells. We found that the Mart-1–specific established effector T cells express both Notch1 and Notch2, whereas resting naive CD8+ T cells only express Notch2. Although the presence of both Notch1 and Notch2 on established effector cells needs to be confirmed in other CD8 T cell clones, these data suggest that Notch2-mediated signaling is crucial during priming of human CD8+ T cells. Alternatively, the difference in effects of Notch signaling on naive versus effector T cells may be caused by differential binding and signaling induced by notch ligands. For murine CD4+ T cells, it was shown that the binding capacity of DLL4 is weaker to Notch receptors on naive T cells than to receptors on activated T cells.36 Whether this also holds true for the other Notch ligands was not investigated. Moreover, these authors showed that the distinct Notch ligands bind differently to antigen-experienced T cells. DLL4 bound Notch receptors stronger than DLL1, whereas Jag1 showed only very weak receptor binding. Because we demonstrated that activated moDCs express both Jag1 and DLL4, it may be possible that these ligands contribute differently to the activation of naive and antigen-experienced CD8+ T cells.
In conclusion, these data indicate that Notch signaling controls activation and effector functions of human CD8+ T cells. This pathway may be targeted to obtain new and more efficacious vaccination strategies for the treatment of cancer.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Michel Kester (Department of Hematology, Leiden University Medical Center [LUMC] Leiden, Netherlands) for providing the Mart-1–specific tetramers.
This work was financially supported by a grant from SenterNovem SII071030 (W.W.J.U. and M.I.V.) and VUmc Cancer Center Amsterdam and VUmc Institute for Cancer and Immunology (CCA/VICI) (L.M.K.),
Authorship
Contribution: L.M.K. designed and performed research and drafted the paper; M.I.V., N.V.R., and S.C.B. performed experiments; E.H., B.O.R., T.D.d.G., and Y.v.K. contributed to discussion, supervised the study, and reviewed and edited the manuscript; and W.W.J.U. designed and supervised research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: W.W.J. Unger, Department of Molecular Cell Biology and Immunology, VU University Medical Center, P.O. Box 7057, 1007 MB, Amsterdam, The Netherlands; e-mail: w.unger@vumc.nl.