To the editor:
In their work Yri et al show that non-Hodgkin lymphoma (NHL) patients undergoing or having received rituximab (anti-CD20 mAb)–containing regimens within the previous 6 months are unable to generate an antibody response to the AS-03–adjuvanted A/H1N1-2009 pandemic influenza vaccine.1
Although the authors conclude that influenza vaccination is not active in this setting, findings obtained by investigating the immunogenicity of a naive antigen [ie, A/California/7/2009(H1N1)pdm09] cannot be thoroughly transposed in the context of seasonal influenza vaccination, which includes recall antigens. In fact, even during the profound rituximab-induced CD20+ B-cell depletion, partial maintenance of humoral immunity to recall antigens is still possible likely because of the persistence of CD20− long-lived plasma cells2,3 and some memory B-cell subpopulations (eg, splenic CD27+IgG+ B cells4 ). Accordingly, in this setting, Takata et al observed a lack of antibody response only to the new antigen (season 2005/2006), not included in previous vaccine compositions.2
To our knowledge, there are no existing data concerning the activity of a naive influenza vaccine beyond the rituximab peri-treatment period. We previously observed a lack of humoral response to trivalent virosomal subunit vaccine associated with prolonged depletion of CD27+ memory B cells in long-standing complete remission (CR) NHL patients (season 2008/2009).5
Here, we evaluated the humoral response to monovalent pandemic MF-59–adjuvanted vaccine containing A/California/7/2009(H1N1)pdm09 antigen (Focetria; Novartis, 2 doses) followed by single-shot trivalent MF-59–adjuvanted seasonal influenza vaccine (Fluad; Novartis) in 14 CR-NHL patients (median age 65 years) well beyond the rituximab peritreatment period in a study approved by the institutional review board (IRB no. MI09.001) of the Istituto Nazionale per la Ricerca sul Cancro (currently IRCCS Azienda Ospedaliera Universitaria San Martino-IST-Istituto Nazionale per la Ricerca sul Cancro, Genoa, Italy). Data were compared with those of 2 cohorts of 14 healthy volunteers (age-matched controls;median age: 67 and 71 years, for the pandemic and the seasonal cohort, respectively) vaccinated with the same pandemic or seasonal vaccination schedule. All the participants were vaccinated during the 2009/2010 season and evaluated at our institutions. Informed consent was provided according to the Declaration of Helsinki. Time between vaccinations was 28 ± 2 days. Hemagglutinin inhibition assay was used to determine antibody titer for each strain (before and 28 ± 2 days after each vaccination).5,6 Seroprotection rate (ie, antibody titer ≥ 40), seroconversion rate (ie, at least 4-fold increase of antibody titer after vaccination), and geometric mean of antibody titer (GMT) were determined.5,6 Patient immunoglobulin levels and B-cell counts were assessed before the first vaccine administration as described.5 B-cell counts were compared with those from 21 healthy volunteers previously assessed in our laboratory.5
Median time after rituximab was 33 months (range = 14-78); concentrations below the lower limit of normal of at least 1 class of immunoglobulins were observed in 6 patients (43%).
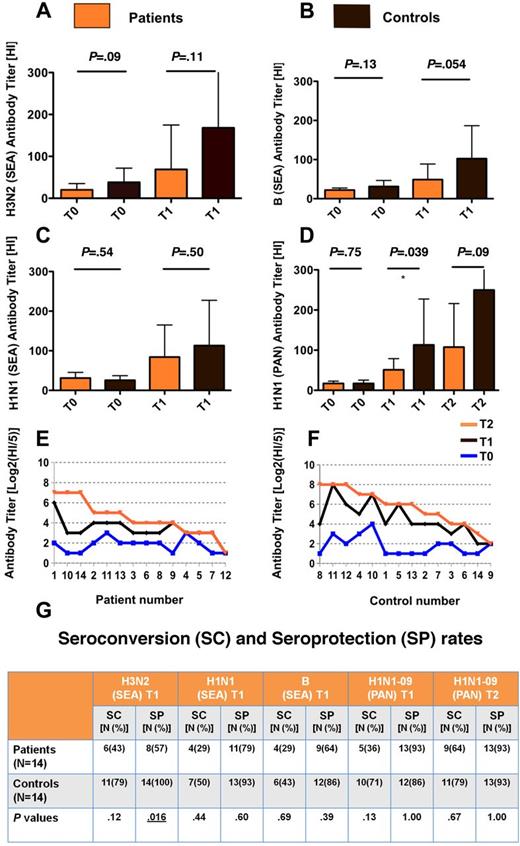
B-cell proportions (CD19+) were superimposable, but CD27+ memory B-cell proportions were extremely low among patients (median = 3%) compared with healthy volunteers (median = 39%; P < .001; 2-sided Mann-Whitney test). The response to influenza vaccination was lower in patients, reaching the statistical significance for GMT against A/California/7/2009(H1N1)pdm09 strain and for seroprotection against A/Brisbane/10/2007 (A/H3N2; Figure 1). Patient seroprotection rates were > 60% for 3/4 strains (Figure 1G). Patient response to A/California/7/2009(H1N1)pdm09 was weak but it was boosted by the second dose. Notably, the 3 subjects who did not respond to the first administration failed the second administration as well (Figure 1E-F).
Postvaccination antibody titers against seasonal and pandemic influenza antigens are lower in patients compared with controls. (A-D) Bar charts showing geometric mean antibody titers (GMT) against seasonal A/Brisbane/10/2007 (H3N2 SEA) antigen, panel A; B/Brisbane/60/2008 (B SEA) antigen, panel B; A/Brisbane/59/2007 (H1N1 SEA) antigen, panel C; and pandemic A/California/7/2009(H1N1)pdm09 (H1N1 PAN) antigen, panel D. Antibody titers in non-Hodgkin lymphoma (NHL) patients (N = 14 for each bar chart) and controls (N = 14 for each bar chart) were assessed before (T0) after 1 dose (T1) of Fluad (seasonal vaccine; panels A-C) and after 1 and 2 doses (T1 and T2, respectively) of Focetria (pandemic vaccine, panel D). Antibody titers were assessed by hemagglutinin inhibition assay. The obtained antibody titer was expressed as the reciprocal of the highest dilution of serum inhibiting hemagglutination. Tests were performed in duplicate. Baseline titers were similar between patients and controls. Postvaccination titers were lower in patients compared with controls. Error bars represent the upper 95% confidence intervals of the geometric means (truncated in the last histograms of the panels A and D; exact values: 399 and 534, respectively). P values are from 2-sided Mann-Whitney tests. (E-F) Single-subject dot charts showing antibody titers against A/California/7/2009(H1N)pdm09 strain at baseline and after 1 and 2 doses of Focetria in patients (N = 14) and controls (N = 14). Data are represented in Log2 scale. In panel E T1 and T2 curves overlapped from patients no. 9 to patients no. 12. The 3 subjects who did not respond at all to the first administration (patients no. 4 and 12, panel E; and control no. 9, panel F) failed the second administration as well. (G) Seroconversion rate and seroprotection rate after seasonal (SEA) and pandemic vaccine in patients compared with controls (P values are from 2-sided Fisher exact test). Six patients (43%) had been diagnosed with aggressive NHL. Twelve patients (86%) received 1 line of chemotherapy and the same proportion had been treated with CHOP or CHOP-like regimens. Three patients (21%) received fludarabine containing regimens. SC indicates seroconversion; and SP, seroprotection.
Postvaccination antibody titers against seasonal and pandemic influenza antigens are lower in patients compared with controls. (A-D) Bar charts showing geometric mean antibody titers (GMT) against seasonal A/Brisbane/10/2007 (H3N2 SEA) antigen, panel A; B/Brisbane/60/2008 (B SEA) antigen, panel B; A/Brisbane/59/2007 (H1N1 SEA) antigen, panel C; and pandemic A/California/7/2009(H1N1)pdm09 (H1N1 PAN) antigen, panel D. Antibody titers in non-Hodgkin lymphoma (NHL) patients (N = 14 for each bar chart) and controls (N = 14 for each bar chart) were assessed before (T0) after 1 dose (T1) of Fluad (seasonal vaccine; panels A-C) and after 1 and 2 doses (T1 and T2, respectively) of Focetria (pandemic vaccine, panel D). Antibody titers were assessed by hemagglutinin inhibition assay. The obtained antibody titer was expressed as the reciprocal of the highest dilution of serum inhibiting hemagglutination. Tests were performed in duplicate. Baseline titers were similar between patients and controls. Postvaccination titers were lower in patients compared with controls. Error bars represent the upper 95% confidence intervals of the geometric means (truncated in the last histograms of the panels A and D; exact values: 399 and 534, respectively). P values are from 2-sided Mann-Whitney tests. (E-F) Single-subject dot charts showing antibody titers against A/California/7/2009(H1N)pdm09 strain at baseline and after 1 and 2 doses of Focetria in patients (N = 14) and controls (N = 14). Data are represented in Log2 scale. In panel E T1 and T2 curves overlapped from patients no. 9 to patients no. 12. The 3 subjects who did not respond at all to the first administration (patients no. 4 and 12, panel E; and control no. 9, panel F) failed the second administration as well. (G) Seroconversion rate and seroprotection rate after seasonal (SEA) and pandemic vaccine in patients compared with controls (P values are from 2-sided Fisher exact test). Six patients (43%) had been diagnosed with aggressive NHL. Twelve patients (86%) received 1 line of chemotherapy and the same proportion had been treated with CHOP or CHOP-like regimens. Three patients (21%) received fludarabine containing regimens. SC indicates seroconversion; and SP, seroprotection.
Thus, CR-NHL patients 14-78 months beyond the last rituximab administration have an attenuated, but not suppressed, response to naive/seasonal influenza antigens, reaching acceptable seroprotection rates. Adjuvanted influenza vaccines should be recommended/offered inthis setting. Two-dose regimens may enhance antibody response although physicians should be aware that completely refractory patients may not benefit from this strategy.
Authorship
Acknowledgments: The authors thank Dr Rocco Iudici, Dr Marisa Alberti, and Dr Pietro Blandini for helping in donor and/or control recruitment, Dr Paola Marroni for performing serologic tests analyses, and Dr Paola Canepa and Dr Antonella Ceravolo for helping in performing HI assays analysis. D.B.'s fellowship is supported by the Conquer Cancer Foundation of the American Society of Clinical Oncology (Young Investigator Award Grant). The present work has been supported by grants awarded by Istituto Superiore di Sanita' (ISS): Programma nazionale di ricerca sull'AIDS, accordi di collaborazione scientifica 45G.1, 40D61, 40H69 (A.D.M.), MIUR, Fondi di Ateneo 2011-2012; and by the NIH Intramural Research Program.
Contribution: D.B., F.A., and A.D.M. designed the research; D.B., F.A., E.Z., P.D., M.R.S, C.M., E.B., O.R., G.Z., A.O., C.A., G.I., S.Z., M.F., and A.D.M. performed the research; D.B., F.A., C.M., S.Z, and A.D.M. analyzed the data; D.B., F.A., S.Z., and F.M.M. contributed vital new reagents and analytical tools; D.B. performed statistical analysis; D.B. and A.D.M drafted the manuscript: D.B., F.A., E.Z., P.D., M.R.S, C.M., E.B., O.R., G.Z., A.O., C.A., G.I., F.M.M., S.Z., M.F., and A.D.M wrote the manuscript; and D.B, F.A., and A.D.M contributed equally to this study.
Conflict-of-interest disclosure: F.A. has previously participated at speakers' bureau and advisory board meetings sponsored by Novartis Vaccines and Sanofi Pasteur and has received research funding from Novartis Vaccines and Sanofi Pasteur. G.I. has previously participated at speakers' bureau and advisory board meetings sponsored by Sanofi Pasteur and has received research funding from Crucell/ISB, GSK and Sanofi Pasteur. P.D. has received financial support for scientific research, speaker's fees and attendances at national and international meetings from influenza vaccine and antiviral manufacturers. P.D. has been and currently is investigator of several clinical trials on innovative vaccines produced by influenza vaccine manufacturers. The remaining authors declare no competing financial interests.
Correspondence: Dr Davide Bedognetti, MD, PhD, Infectious Disease and Immunogenetics Section, Department of Transfusion Medicine, Clinical Center, National Institutes of Health, 10 Center Dr, Rm 1N224, Bethesda, MD, 20892; e-mail: davide.bedognetti@nih.gov or bedodav@yahoo.it; or Prof Andrea De Maria, MD, SS Infettivologia, IRCCS Azienda Ospedaliera Universitaria San Martino-IST-Istituto Nazionale per la Ricerca sul Cancro, Largo Rosanna Benzi 10, 16132 Genoa, Italy; e-mail: de-maria@unige.it.