Abstract
Coagulation factor XII (FXII, Hageman factor, EC = 3.4.21.38) is the zymogen of the serine protease, factor XIIa (FXIIa). FXII is converted to FXIIa through autoactivation induced by “contact” to charged surfaces. FXIIa is of crucial importance for fibrin formation in vitro, but deficiency in the protease is not associated with excessive bleeding. For decades, FXII was considered to have no function for coagulation in vivo. Our laboratory developed the first murine knockout model of FXII. Consistent with their human counterparts, FXII−/− mice have a normal hemostatic capacity. However, thrombus formation in FXII−/− mice is largely defective, and the animals are protected from experimental cerebral ischemia and pulmonary embolism. This murine model has created new interest in FXII because it raises the possibility for safe anticoagulation, which targets thrombosis without influence on hemostasis. We recently have identified platelet polyphosphate (an inorganic polymer) and mast cell heparin as in vivo FXII activators with implications on the initiation of thrombosis and edema during hypersensitivity reactions. Independent of its protease activity, FXII exerts mitogenic activity with implications for angiogenesis. The goal of this review is to summarize the in vivo functions of FXII, with special focus to its functions in thrombosis and vascular biology.
The factor XII–driven plasma contact system
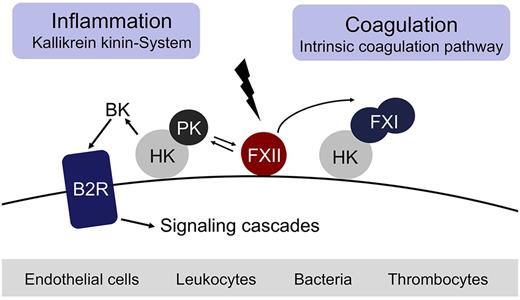
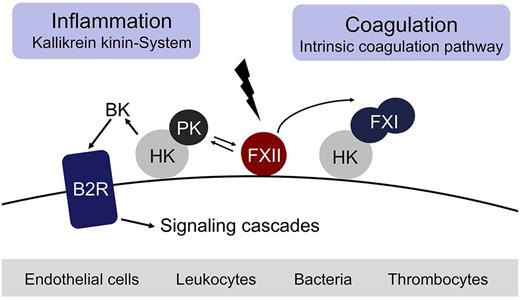
Fibrin formation may be initiated by 2 distinct pathways, either triggered by exposure of blood to a damaged vessel wall (extrinsic) or to blood-borne (intrinsic) factors. The intrinsic pathway of coagulation is initiated by factor XII (FXII, Hageman factor), in a reaction involving high molecular weight kininogen (HK) and plasma kallikrein (PK). These factors are collectively referred to as the plasma contact system.1-6 Contact with negatively charged surfaces induces a conformational change in zymogen FXII resulting in a small amount of active FXII (FXIIa).7 FXIIa cleaves PK to generate active PK, which in turn reciprocally activates FXII.8 FXIIa triggers fibrin formation through activation of factor XI (FXI) and also liberates the inflammatory mediator bradykinin (BK) from HK through cleavage by PK.3 Binding of BK to the kinin B2 receptor (B2R) activates proinflammatory signaling pathways that dilate vessels, induce chemotaxis of neutrophils, and increase vascular permeability.9 Thus, the FXIIa-driven contact system has proinflammatory and procoagulant activities via the kallikrein kinin-system and the intrinsic coagulation pathway, respectively (Figure 1). The serpin C1 esterase inhibitor (C1INH) is the major plasma inhibitor of FXIIa and PK and controls proteolytic activity of the contact system.10 Besides C1INH, antithrombin III (ATIII) and PAI-1 also have FXIIa-blocking activity.11 In vitro, FXIIa triggers activation of the classic complement pathway and initiates the fibrinolytic system via PK-mediated urokinase activation.5 Whether FXIIa has the capacity to trigger activation of the complement and fibrinolytic systems in vivo remains uncertain.
The FXII-driven contact system. Contact with negatively charged surfaces activates coagulation FXII on endothelial cells, leukocytes, bacteria, and thrombocytes and initiates procoagulant and proinflammatory proteolytic reactions. Activated FXII triggers fibrin formation through the FXI-mediated intrinsic pathway of coagulation. Simultaneously, activation of prekallikrein by FXIIa leads to generation of the vasoactive peptide BK by PK-mediated cleavage of HK.
The FXII-driven contact system. Contact with negatively charged surfaces activates coagulation FXII on endothelial cells, leukocytes, bacteria, and thrombocytes and initiates procoagulant and proinflammatory proteolytic reactions. Activated FXII triggers fibrin formation through the FXI-mediated intrinsic pathway of coagulation. Simultaneously, activation of prekallikrein by FXIIa leads to generation of the vasoactive peptide BK by PK-mediated cleavage of HK.
Factor XII is dispensable for hemostasis
The enzymology of the FXII-driven contact system in vitro is well understood. However, its in vivo contributions are just beginning to emerge. FXII-contact activation in vitro provides the mechanistic basis for one of the most commonly used diagnostic coagulation tests, the activated partial thromboplastin time (aPTT), which is extensively used in clinical practice (> 500 million assays/per year worldwide) for preoperative screening, the diagnostics of thrombosis-related autoimmune diseases, and monitoring of anticoagulation therapy. Despite its contribution to fibrin formation in vitro, FXII-initiated coagulation in vivo was not considered to be of significance. This premise is based on the observation that FXII-deficient persons and animals do not exhibit a clinically relevant bleeding phenotype: persons with partial or severe FXII deficiency do not bleed excessively from sites of injury despite a marked prolongation of the aPTT.12,13 This apparent discrepancy between the essential role of FXII for contact-driven fibrin formation in test tubes that eventually lacks a correlation in vivo puzzled investigators for decades. Similar to FXII deficiency, persons lacking the contact proteins PK or HK do not have impaired hemostasis and are commonly diagnosed during routine coagulation screening when a prolonged aPTT is discovered. In contrast, patients deficient in FXI have a mild trauma-induced bleeding disorder (sometimes called “hemophilia C”) that is mostly restricted to tissues with high fibrinolytic activity. Severe FXI deficiency is a rare inherited abnormality in the general population (seen with a 1 in a million people prevalence), but is more common in specific populations, such as Ashkenazi Jews (1 in 450).14
This lack of a bleeding tendency observed with FXII deficiency is in sharp contrast to deficiencies of other components of the coagulation cascade, such as FVII, tissue factor (TF) and FVIII or FIX (causing the bleeding disorders hemophilia A and B, respectively) and has led to the reasonable hypothesis that fibrin formation in vivo is initiated largely, if not exclusively, through the extrinsic pathway of coagulation. Notably, complete ablation of TF expression causes embryolethal intrauterine bleeding in mice. Human TF deficiency has not been described, indicating that TF is essential for development and/or survival.15 The dominant role of VIIa/TF-driven coagulation for hemostasis is supported by the “revised model of coagulation,” which shows that FXI, the substrate of FXIIa during contact-initiated clotting, can also be activated by thrombin, independently of FXII.16 Although FXII is dispensable for fibrin formation in hemostatic reactions, severe deficiencies (< 10% of normal plasma levels) in the coagulation protein are invariably rare,17 suggesting that FXII has critical roles that warrant future studies. Interestingly, FXII is a rather modern protein in terms of evolution. Copies of the FXII gene are absent in inframammalian vertebrates, such as birds or fish,18 which have a closed circulatory system, supporting the notion that FXII is not required to seal vessel injuries.
Factor XII has an essential contribution to thrombosis
Injury to a blood vessel triggers activation of blood platelets and the plasma coagulation system, leading to formation of a blood clot consisting of platelets and fibrin. To investigate the role of FXII in vivo, FXII−/− mice were generated and characterized in experimental thrombosis models.19,20 Identical to FXII-deficient humans, FXII−/− mice have a normal hemostatic capacity as assessed by a tail-bleeding assay and could undergo surgical procedures without excessive bleeding.21 Completely unexpected, intravital fluorescence microscopy and blood flow measurements in 3 distinct arterial beds revealed a severe defect in thrombus formation in FXII-deficient mice when challenged by chemical (FeCl3), mechanical, and Rose Bengal/laser of vascular injury models.21,22 The thrombo-protective phenotype of FXII−/− mice in combination with their normal hemostatic capacity challenges the previously accepted notion of a “coagulation balance.” Although FXII has a crucial role in fibrin formation during “pathologic” thrombosis, it does not contribute to “physiologic” hemostatic fibrin formation at sites of vessel injury. Reconstitution of FXII−/− mice with human FXII normalized both their prolonged aPTT and the defective thrombotic response. The reconstitution experiments indicate that FXII operates similarly in mice and humans. Indeed, the contact system is highly conserved among mammalian species.23 Thrombus formation in FXII heterozygous mice (having 50% of normal FXII plasma levels) was similar to wild-type animals (having 100%), indicating that half of normal plasma concentration is sufficient for vessel-occlusive clot formation. Modulating FXII expression levels by antisense nucleotides showed that reduction of more than 75% FXII antigen plasma levels is required to produce thrombo-protection in a model of stasis-induced venous thrombosis.24
The decisive role of FXII for experimental models of thrombus formation extends to thromboembolic disease: FXII-deficient mice are protected from cerebral ischemia in an experimental stroke model.25 These thrombo-protective effects are conferred by an impaired FXII-dependent fibrin formation in the microvasculature of the ischemic tissue. Indeed, mice lacking FXI are similarly protected from vessel-occlusive fibrin formation, suggesting that FXII impacts on pathologic clotting predominantly via the intrinsic pathway.26,27 This notion is supported by observations that, in models of lethal pulmonary embolism, survival of mice with combined deficiency in FXII and FXI (FXII−/−/FXI−/−) is similar to animals with deficiency in FXII or FXI alone.28 Given the results from mouse models, the concept that pathologic thrombus formation represents a disequilibrium between the enzymatic reactions that produce a clot at a site of vascular injury probably needs revision. Indeed, pharmacologic inhibition of FXII activity may provide an attractive approach for the management of thrombotic disease. For instance, the peptide-based inhibitor PCK (Phe-Pro-Arg-chloromethylketone) provides protection from cerebral ischemia in wild-type mice without causing excessive bleeding at a site of surgical injury.25 Consistently, a recombinant infestin-4–based inhibitor that specifically targets FXIIa, provides protection from cerebral ischemia in experimental stroke models, albeit it did not reduce the hemostatic capacity of inhibitor-treated mice.29 Cumulatively, the findings in animal models raise the exciting possibility that targeting FXII may offer a safe and powerful strategy for prevention or treatment of pathologic thrombosis without the associated risk of hemorrhage that accompanies currently used anticoagulants.30,31 However, the detailed association of FXII deficiency and risk for thrombosis is probably more complex. The instability of arterial thrombi observed in FXII-deficient mice,21 defective polyphosphate (polyP)–driven fibrin formation in FXII-deficient human and mouse plasma,28,32 as well as case reports of pulmonary emboli seen in FXII-deficient persons33 raises an intriguing hypothesis: FXII deficiency may protect from arterial thrombosis as high shear stress interferes with propagation of unstable thrombi into the vessel lumen. In contrast, larger thrombi may develop in veins under low shear conditions. However, reduced stability of these thrombi increases their risk to embolize.
Analysis of a large registry involving approximate 9000 patients from Austria supports this dual function of FXII in thrombosis. There is an inverted U-shaped association of FXII plasma levels and overall mortality and mortality from cardiovascular disease.34 Clearly more epidemiologic studies are necessary to define the precise consequences of FXII deficiency in arterial and veno-occlusive disease.
Mechanisms of contact activation in vivo
FXII activation by nonphysiologic negatively charged surfaces, such as glass, is well established. Various materials have been identified that have the potency of triggering FXII activation.35 Among the best-characterized agents that initiate FXII-dependent clotting are the white clay minerals kaolin and celite (silica-rich compounds), which are commonly used in aPTT coagulation assays. Moreover, kaolin is also used as a hemostatic agent in vivo to terminate blood loss after injury in combat victims. Although the idea of using FXII activators as hemostatic agents for sealing injuries is attractive, exposure of flowing blood to kaolin triggers thromboembolic events and produces excessive heat at the wound site, resulting in additional burn injury and subsequent tissue necrosis.36
Initiated by the discovery that FXII is crucial for occlusive thrombus formation,21 there was a renewed search for candidate endogenous activators of this protease. RNA that is released from injured cells was shown to activate FXII in plasma. Administration of RNAse (an enzyme that degrades RNA) to mice before experimental challenge has thrombo-protective effects in a FeCl3-induced carotid artery injury model in mice.37 RNA is released from various types of disintegrating cells. The regulatory mechanisms that allow RNA-driven FXII in thrombosis, but not at injury sites at the vessel wall, remain to be identified. However, oligonucleotides, such as RNA, and nucleotide-associated proteins, such as histones,38 contribute to procoagulant states associated with infections and sepsis. FXII binding to collagen is known to enhance coagulation in vitro.39 This interaction is dependent on repetitive negative charge exposed by collagen fibrils.40 When added to platelet-rich plasma, equine type I collagen (Horm) promotes thrombus formation under flow in an FXII-dependent manner.41 In contrast, FXII activity does not contribute to thrombus formation under flow over atherosclerotic plaque material.42 The presence of platelets dramatically enhanced the FXII-dependent procoagulant capacity of collagen,41 suggesting that it also promotes FXII activation indirectly through release of platelet-derived activators.28 In line with this observation, it has been found that activated platelets support FXII activation in a mechanism that is dependent on integrin αIIb-β3 signaling.43 Moreover, activated platelets support activation of the kallikrein-kinin system,44 as well as fibrin formation in an FXII-dependent manner,45 even in the presence of thrombin and TF inhibition.44,46 Taken together, these studies link platelet activation and FXII-driven fibrin formation and raise the question: how do procoagulant platelets activate FXII?
Polyphosphate drives FXII activation during thrombosis
Platelet polyP is an inorganic polymer that is released on platelet activation. Polyphosphate was originally identified in nonmammalian cells and is highly enriched in platelet dense granules.47 Synthetic polyP is used as water softener in technical processes and has been shown to modulate plasma coagulation by multiple mechanisms involving FXII,28 FXI,48 the fibrinolytic system,49 factor V,50 and through alterations of the fibrin structure49 in ex vivo experiments.51 Platelet polyP is a nonbranched polymer of 60-100 orthophosphate residues that directly binds to FXII (and HK) with high affinity.28 Polyphosphate potently initiates coagulation in human plasma and in mice in an FXII-dependent manner. Targeting polyP with phosphatase (an enzyme that cleaves phosphoester bonds and degrades polyP) largely abolishes the formation of occlusive thrombus formation in vivo. Conversely, synthetic polyP restored defective clot formation in platelet-rich plasma from Hermansky-Pudlak patients, who (among others) lack the storage pool for platelet polyP,28 suggesting that polyP may be used as a hemostatic agent. Together, these findings identify polyP as the endogenous platelet-derived FXII activator in vivo.52 While triggering FXII-dependent clotting, polyP/FXII also activates the kallikrein-kinin system leading to PK-mediated BK formation.28 Cumulatively, the identification of polyP as a platelet-derived procoagulant agent provides a long-sought link between primary and secondary hemostasis41 and may represent a new paradigm for the treatment of thromboembolic and inflammatory diseases.53
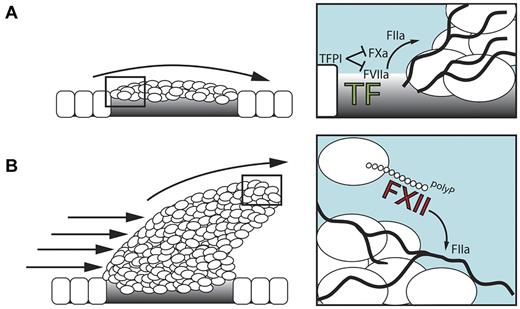
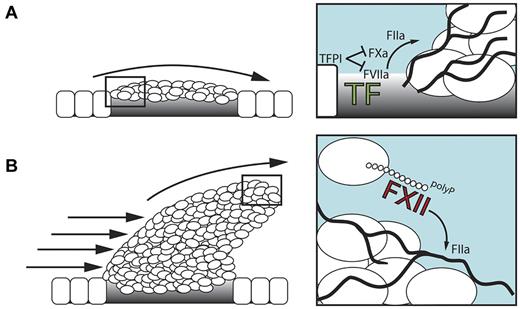
TF exposed at subendothelial sites of vessel injury initiates fibrin production. However, tissue factor pathway inhibitor that is released from platelets and endothelial cells rapidly terminates TF activity (Figure 2A). In addition, adherent platelets shield the transmembrane TF protein at the injury site. There is a requirement to stimulate ongoing fibrin production within the 3-dimensionally developing thrombus. polyP released by procoagulant platelets triggers FXII activation and drives fibrin formation via the intrinsic coagulation pathway (Figure 2B). Deficiency in platelet polyP-stimulated FXII-driven fibrin formation, interferes with fibrin fiber thickness,32,49 resulting in mechanical clot instability28 and probably increases the risk of thrombus embolization. Blood-borne TF on circulating monocytes, neutrophils,54 and microparticles,55 which are incorporated into the developing thrombus in a P-selectin–dependent manner,56 provides an alternative FXII-independent mechanism for triggering fibrin production. The relative importance of TF/FVIIa- versus polyP/FXII-initiated fibrin formation and their contribution to thrombosis has not been directly compared and may differ depending on vascular beds and types of vessel injury.
The role of polyP/FXII in thrombosis. (A) Initially, the TF/FVIIa-driven “extrinsic” coagulation pathway triggers fibrin formation at sites of injury. FXII has no function during this stage. Tissue factor pathway inhibitor (TFPI) is released from endothelial cells and adherent platelets and blocks TF activity. (B) In the developing thrombus, activated platelet–released polyP triggers fibrin production via activation of FXII that drives the “intrinsic” coagulation cascade. polyP/FXII-driven fibrin formation operates distant from the injured vessel wall and, hence, does not contribute to hemostasis.
The role of polyP/FXII in thrombosis. (A) Initially, the TF/FVIIa-driven “extrinsic” coagulation pathway triggers fibrin formation at sites of injury. FXII has no function during this stage. Tissue factor pathway inhibitor (TFPI) is released from endothelial cells and adherent platelets and blocks TF activity. (B) In the developing thrombus, activated platelet–released polyP triggers fibrin production via activation of FXII that drives the “intrinsic” coagulation cascade. polyP/FXII-driven fibrin formation operates distant from the injured vessel wall and, hence, does not contribute to hemostasis.
Factor XII zymogen as a growth factor
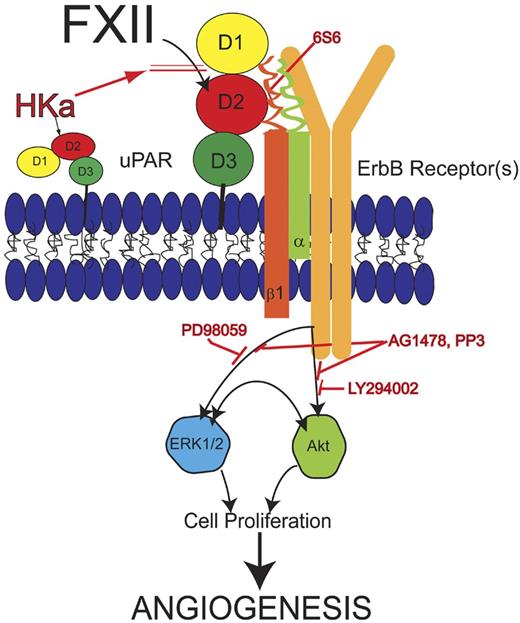
Although most investigations focus on FXII as a serine protease, zymogen FXII has mitogenic activities on cultured cells independent of its enzymatic activity. FXII's heavy chain consists of a fibronectin type II and I domain, 2 EGF-like domains, a kringle domain, and a proline-rich region adjacent to its catalytic domain.57 The EGF-like domains in FXII, like those in single-chain urokinase and tissue-type plasminogen activator share structural similarities with homologous domains in hepatocyte growth factor.58 Both FXII and FXIIa are mitogenic on cultured HepG2 cells and phosphorylate MAPK in HepG2 and vascular smooth muscle cells.59 FXII zymogen stimulates 5-bromo-2′-deoxyuridine incorporation through the ERK1/2 and Akt S473 phosphorylation pathway in endothelial cells in a uPAR-dependent manner.60,61 The domain 2 region of uPAR where FXII binds is a regulatory site of the uPAR interactome. In addition to FXII ScuPA, HK/HKa, vitronectin, and PAI-1/uPA, bind to this region to overlapping, but not identical sequences, on uPAR's domain 2 as well. Consistently, FXII stimulates aortic sprouts from wild-type mice but not from uPAR−/− aorta and initiates new vessel formation into Matrigel plugs in wild-type but not in uPAR-deficient animals. Vice versa, there is less number of vessels in skin punch biopsies in a FXII−/− mouse model both constitutively and in a wound healing model.62 In contrast, in another FXII−/− mouse strain, there are no obvious vascular abnormalities in histologic analyses.19
These combined data indicate that zymogen FXII, like single-chain urokinase, functions as a growth factor that mediates cell signaling leading to proliferation and stimulating angiogenesis, indicating a new in vivo activity for zymogen XII in postnatal angiogenesis after ischemia, inflammation, and injury (Figure 3).
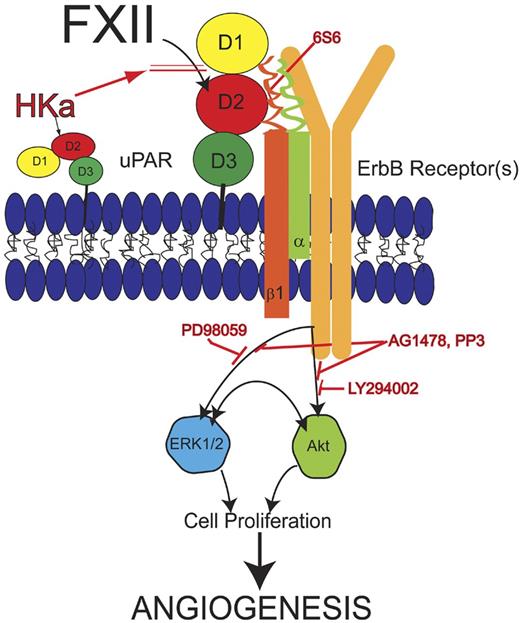
Model of zymogen FXII signaling pathway. FXII binds to domain 2 of uPAR and induces uPAR to communicate intracellularly through β1 integrins. Monoclonal antibody 6S6 to β1 integrin blocks this pathway. Cell stimulation through uPAR and integrin requires an interaction with 1 or more of the ErbB receptor kinases because the tyrosine inhibitors AG1478 or PP3 block FXII signaling. The MEK inhibitor PD98059 blocks FXII-induced ERK1/2 phosphorylation. LY294002, a PI3 kinase inhibitor, blocks FXII-induced Akt phosphorylation. Crosstalk between pERK1/2 and pAkt systems also occurs. Cleaved forms of HK (HKa) block binding of FXII to endothelial cells. Inhibition of any step of the FXII signaling pathways blocks cell proliferation and angiogenesis in HUVEC and aortic segments, respectively. Modified from Falati et al56 with permission.
Model of zymogen FXII signaling pathway. FXII binds to domain 2 of uPAR and induces uPAR to communicate intracellularly through β1 integrins. Monoclonal antibody 6S6 to β1 integrin blocks this pathway. Cell stimulation through uPAR and integrin requires an interaction with 1 or more of the ErbB receptor kinases because the tyrosine inhibitors AG1478 or PP3 block FXII signaling. The MEK inhibitor PD98059 blocks FXII-induced ERK1/2 phosphorylation. LY294002, a PI3 kinase inhibitor, blocks FXII-induced Akt phosphorylation. Crosstalk between pERK1/2 and pAkt systems also occurs. Cleaved forms of HK (HKa) block binding of FXII to endothelial cells. Inhibition of any step of the FXII signaling pathways blocks cell proliferation and angiogenesis in HUVEC and aortic segments, respectively. Modified from Falati et al56 with permission.
Role of FXII in human thrombotic disease
A severe human FXII deficiency is rare and, as a consequence, there is a lack of epidemiologic studies that systematically compare the incidence or severity of thromboembolic events (ie, stroke, myocardial infarction, pulmonary embolism) in humans with low FXII plasma levels. Based on the animal studies, one would expect that FXII deficiency would protect from thrombosis in patients. In contrast, there is a long history of anecdotal reports suggesting that FXII deficiency may actually predispose to thrombosis.63,64 FXII deficiency associated with increased risk of thrombosis dates to the death of the index patient with FXII deficiency, John Hageman, who died of a pulmonary embolism.65 In brief, railroad worker Hageman fell from a boxcar and fractured his left hemipelvis. He was kept at bed rest for a week and was subsequently allowed to walk on crutches. A few days later, he was found gasping for breath, pulseless, and passed away within minutes. Autopsy showed massive thrombi occluding his left and right pulmonary arteries and several large thrombi were recovered, which presumably had originated from his lower extremity veins.65 It is difficult to link FXII deficiency to this lethal pulmonary embolism event because the trauma and subsequent immobilization represent established FXII-independent risk factors for venous thrombosis. Indeed, careful reanalysis has identified other risk factors in FXII-deficient patients with thrombosis, arguing against FXII deficiency as an independent prothrombotic risk factor.66 Consistently, larger epidemiologic studies in The Netherlands and Switzerland did not find a correlation between FXII deficiency and increased thrombotic risk. However, none of these studies had analyzed whether FXII deficiency conferred thrombo-protection.67,68 In contrast, recent clinical studies from Israel have analyzed the incidence of ischemic stroke and deep vein thrombosis in humans with severe deficiency of FXI (the direct substrate of FXIIa in the intrinsic coagulation pathway). Similarly to FXI-null mice, FXI-deficient humans are largely protected from cerebral ischemia69 and venous thrombosis,70 supporting the decisive role of the intrinsic coagulation pathway for thrombosis in humans.
Hereditary angioedema
FXII has the capacity of activating the classic complement pathway in plasma.71 A simultaneous activation of the contact and complement system often occurs in pathologic conditions. Hereditary angioedema (HAE [MIM #106100]) is a life-threatening tissue swelling disorder that develops in persons who are quantitatively or qualitatively deficient in C1-esterase inhibitor (C1INH; HAE type I and II, respectively). C1INH deficiencies facilitate excessive activation of the FXII-driven complement and contact system cascades and the development of edema in HAE type I and II patients.72 In addition to these 2 well-known HAE types, a third variant exists that almost exclusively affects women. HAE type III patients have normal levels of fully functional C1INH but have angioedema nonetheless.73 Clinically, all types of HAE are characterized by recurrent episodes of acute swelling involving the skin, oropharyngeal, laryngeal, or gastrointestinal mucosa. The pathophysiology of the observed increased vascular permeability in HAE has remained controversial. Elegant studies with genetically modified mice demonstrated that edema formation in C1INH-dependent HAE forms is the result of pathologic contact system activation.74 Genetic ablation of C1INH expression results in excessive BK production and excess BK signaling which increases vascular permeability in humans75 and mice.74 In contrast, in combined C1INH and kinin B2 receptor gene-deficient mice vascular leak is normal. Comprehensive studies have identified BK as the principal mediator of vascular leakage in HAE-related swelling attacks in patients.76 Hence, HAE types I and II are treated by infusion of C1INH77 or kinin B2-receptor antagonists (Icatibant).78 Alternatively, recombinant PK inhibitors (Ecallantide) may be used to interfere with acute swelling episodes in HAE patients.79 In contrast to C1INH-dependent forms of HAE, the disease mechanism of HAE type III was enigmatic. Using genome-wide linkage analyses in affected families, HAE type III was shown to be an autosomal dominant disease associated with a single missense mutation (c.1032CrA) in the gene of FXII.80 Consecutive independent studies involving other families found HAE type III to be associated with a different mutation affecting the same nucleotide of the FXII gene, c.1032CrG.81 Both point mutations translate into amino acid exchanges Thr328Lys and Thr328Arg (numbering includes the signal peptide), respectively, on the protein level. The aPTT assay yields normal values in all types of HAE patients and fails to detect affected persons. FXII plasma levels in HAE type III patients are in the normal range,80 suggesting that a yet unknown mechanism triggers edema predominantly in women. HAE patients experience recurrent attacks of swelling, but the stimuli that trigger these periodic episodes of excessive vascular leakage are poorly defined.72
Mast cell–mediated activation of FXII
Until recently, it was thought that mast cell–mediated vascular leakage is predominantly, if not exclusively, mediated by the release of histamine and targeting histamine signaling is widely used therapeutically to treat edema formation associated with aberrant mast cell activity.82 In allergic disease, BK is generated and contributes to increased vascular permeability.78,83,84 Mast cells are highly effective sentinel cells that are found close to blood vessels and are especially common at sites of potential infection.85 A hallmark of mast cell activity in host defense and allergic reactions is increased vascular permeability. In addition to histamine, mast cell secretory granules also contain highly sulfated polysaccharides with heparin as a major constituent. This glycosaminoglycan is synthesized exclusively by mast cells86 and has been identified as a FXII contact activator in vitro.87 Heparin released from allergen-activated mast cells initiates formation of BK in a FXII-dependent manner.88 Minute amounts of heparin (≥ 4μg/mL) are sufficient to activate FXII. FXI is not activated under these circumstances, suggesting the presence of a regulatory mechanism for plasma prekallikrein-directed activity of FXIIa. Heparin also protects FXIIa from inhibition by C1INH.89
Intravital confocal laser scanning microscopy and tracer extravasation experiments identified BK as the active mediator for increasing leakage in heparin-driven edema in the skin.88 From mast cell–mediated hypersensitivity reactions in mouse models, it was estimated that heparin-driven BK formation accounts for a significant portion (∼ 50%) of total mast-cell evoked increase in vascular permeability. Consistently, small-molecule inhibitors of FXIIa or kinin B2 receptors both interfere with experimental mast cell-triggered leakage. Based on these experimental findings, targeting heparin-initiated BK formation may represent a promising strategy to counteract aberrant mast cell activation in a broad variety of diseases.
Contaminated heparin
The identification of mast cell heparin as an endogenous FXII contact activator in hypersensitivity reactions is reminiscent of reports that had associated therapeutic heparin infusion and contact system activation in a series of life-threatening complications. For decades, heparin has been widely used as an anticoagulant drug. This polysaccharide prevents the formation and extension of blood clots in the circulatory system by increasing AT III activity. Starting November 2007, there was a dramatic increase in heparin-induced adverse reactions in the United States and Germany, such as lethal acute hypersensitivity reactions in patients intravenously receiving commercial heparin of specific lots from a single manufacturer (http://www.fda.gov/cder/drug/infopage/heparin/adverse_events.htm). Consecutively, more than 150 patients died from anaphylactic hypotension associated with intravenous heparin treatment. Comprehensive analyses identified a non-natural contaminant occurring in suspect preparations of heparin that was characterized as oversulfated chondroitin sulfate (OSCS).90 OSCS-contaminated heparin has a greatly increased potency for activating FXII and triggering PK-mediated BK formation in human plasma and in a model of experimental hypotonic shock in vivo.91 These catastrophic reactions in patients are reminiscent of experimental shock models induced by dextran sulfate (DXS)–stimulated BK formation in pigs. Infusion of high-molecular weight DXS (500 kDa) induced transient systemic hypotension,7 and Icatibant (an antagonist of the kinin B2 receptor) blocked this effect on blood pressure.92 The FXII-activating property seems to be dependent on negative charge density of the polysaccharide rather than on a defined structure. Indeed, potency of FXII-driven contact activation in a reconstituted system decreased from dextran sulfate and OSCS (with an average 4 sulfate residues per disaccharide),7,93 to mast cell heparin (with an average of ∼ 2.7 sulfate residues per disaccharide),87 whereas heparan sulfate (with an average of ∼ 1 sulfate residue per disaccharide) was inactive. The potency to activate the plasma contact system also greatly varies among diverse heparin preparations,94 reflecting differences in purification procedures, sources of the polysaccharides, and experimental settings. Of note, although intravenous heparin infusion may trigger BK generation, infusion of the polysaccharide, even at high concentrations in a bolus, normally does not induce hypotension or cause edema. BK that is generated in venous vessels is rapidly and almost completely degraded by angiotensin-converting enzymes and other kininases that are abundantly expressed in lung microvessels before reaching precapillary vascular beds, which regulate blood pressure.
Differential activities of FXII
DXS is a polysulfated polysaccharide of linked glucose moiety. DXS-mediated FXII activation is critically dependent on the chain-length and degree of sulfation of the polyanion.7 High molecular weight DXS (500 kDa) is a potent stimulator of FXII activation, whereas shorter DXS polymers fail to independently activate FXII but do support cleavage of FXII by PK.95 Although long-chain DXS induces BK-mediated hypotension in vivo,92 it does not trigger intravascular coagulation. This indicates that some FXII activators do not have the capacity of triggering coagulation and reveal selectivity in the responses to FXII contact-activating surfaces. Indeed, several FXII contact activators initiate unilateral activation of the kallikrein-kinin system. For instance, other polysaccharides besides DXS, such as OSCS or heparin, specifically initiate BK formation without triggering a procoagulant activity. Misfolded protein aggregates, the toxic protein species among others found in the cerebrospinal fluid of Alzheimer disease patients and the plasma of patients with amyloidosis, trigger FXII activation.96 These hazardous protein aggregates specifically initiate BK formation via activation of the kallikrein-kinin system but do not trigger activation of the intrinsic pathway of coagulation. The mechanism for selective activation of PK without activation of homologous FXI97 is not entirely clear but might reflect higher plasma concentrations of PK as of FXI. Furthermore, increased affinity of heparin-dependent plasma inhibitors, such as antithrombin III (AT III) for activated FXI versus PK, might direct FXII activation driven by charged polysaccharides to BK formation.91 Notably, both PK and FXI are surface-bound via HK and share a conserved HK-binding site.97-99 In addition, surface characteristics of the contact activator might be decisive for PK and/or FXI activation. FXII binds differently to negatively charged surfaces, such as polyP, compared with its binding to misfolded protein aggregates. Anionic surface binding is thought to be mediated through the type II fibronectin domain, the second EGF domain, and kringle domain,100,101 whereas interaction with misfolded protein aggregates is mediated by the fibronectin type I domain.102 Differences in FXII binding may also modulate the conversion of α-FXIIa to β-FXIIa by kallikrein activity and shift FXII activity toward BK formation.103,104
Future perspectives
The discovery that pathologic thrombus formation in FXII−/− mice is largely defective in models for experimental arterial thrombosis and ischemic stroke has created a new interest in this protein, especially because it raises the possibility of treating thrombosis without compromising hemostasis. Recently, several in vivo contact activators of FXII have been identified, including platelet polyP, oversulfated chondroitin sulfate, nucleotides, misfolded protein aggregates, and mast cell heparin. These various activators may be involved in the development of thrombotic and/or inflammatory diseases. In addition, investigations on the growth factor function of FXII have only begun. Cumulatively, these discoveries may help elucidate the physiologic function(s) of FXII in vivo, which has remained persistently mysterious since the protein was discovered more than 50 years ago. Further investigations will expectedly reveal novel roles of FXII through which certain disease states, such as thrombosis, inflammation, and infections, can be therapeutically modified.
Acknowledgments
This work was supported in part by Vetenskapsrådet (K2010-64X-21462-01-3), Hjärt Lungfonden (20090642 and 20110500), Stockholms läns landsting (ALF 20090540 and 2110471), Cancerfonden (100615), and the Federal Ministry of Education and Research (01EO1003 program and the StG-2012-311575; T.R.). A.H.S. was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (R01 HL052779-15 and R21 HL112666-01). C.M. was supported by The Netherlands Organization for Scientific Research (Veni fellowship 016-126-159).
National Institutes of Health
Authorship
Contribution: T.R., A.H.S., K.F.N., M.B., and C.M. wrote the review and discussed its contents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Renné, Department of Molecular Medicine and Surgery, Karolinska Institutet, Karolinska University Hospital Solna (L1:00), SE-171 76, Stockholm, Sweden; e-mail: thomas@renne.net.