Abstract
Abstract 4107
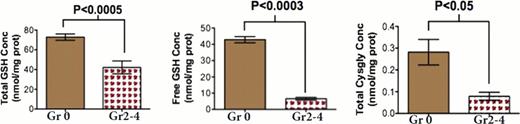
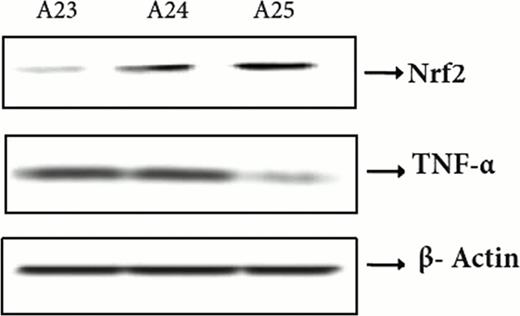
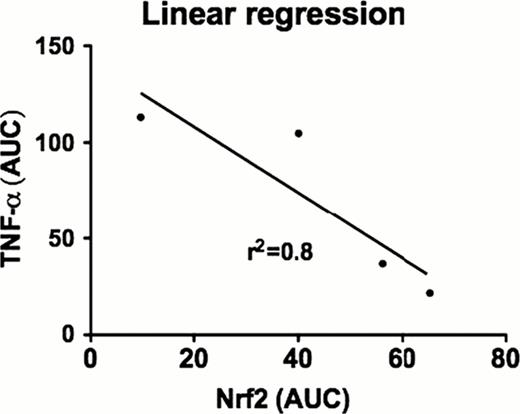
Graft-versus-host disease (GVHD) is characterized by cytokine and chemokine dysregulation that predicts and also contributes to disease severity. Intermediates in sulfur amino acid (SAA) metabolism are known to modulate cytokines implicated in GVHD, such as TNF-α and IL-2. In this report, a new redox metabolomics assay was used to simultaneously profile 40 SAAs and other amino acid metabolites in plasma and liver 0, 4, and 10 days following experimental allogeneic (Balb/C→ B6, Allo) and syngeneic ((B6Thy1.1→ B6, Syn) bone marrow transplantation (BMT). At day 4 post transplantation (Day +4), prior to increase in plasma TNF-α and subsequent GVHD histopathology, there was a significant decline in both plasma and hepatic glutathione (GSH), resulting in a more oxidized redox potential in Allo relative to Syn mice. Specifically, at Day +4, plasma free GSH concentrations in Allo mice were ∼65% lower than Syn controls (p<0.005), and were accompanied by a ∼3-fold increase in plasma GSSG (p=0.012). The elevated GSSG/GSH ratio indicates a significant shift toward a more oxidized state. Loss of GSH was not due substrate limitation because plasma concentrations of total cysteine, the rate-limiting substrate for GSH synthesis, were significantly higher in Allo vs Syn mice at Day +10, indicating an allo-specific block in GSH synthesis. The plasma GSH redox potential on both Day +4 and Day +10 was significantly lower in Allo (−116.7 ± 5.8 mV, −129.0 ± 11.3 mV, respectively) compared to Syn (−157.9 ± 6.7 mV, −158 ± 2 mV) mice, in which the steady-state GSH redox potential remained similar to values in wild-type controls. Early GSH depletion appears to be a metabolic event associated solely with GVHD pathology, and not a consequence of effects of the conditioning regimen, as shown by the striking difference in hepatic GSH redox potential in an unconditioned paternal to F1 hybrid transplantation model (B6 → B6D2F1) in which some mice develop GVHD (Allo/GVHD+) and some do not (Allo/GVHD-). Specifically, the GSH/GSSG ratio was significantly higher in the Allo/GVHD-compared to Allo/GVHD+ group (17.0 ± 4.91 vs 0.315 ± 0.0601, p=0.015). At day+10, Allo/GVHD+ and Allo/GVHD-mice could be segregated based on hepatic total GSH, free GSH and total cystinylglycine concentrations (Figure 1). Increase in plasma TNF-α lagged behind the redox changes, with no significant differences detected between Syn and Allo mice at Day +4. Also observed at Day +4 in Allo mice was a significant decline in the hepatic nuclear concentration of NF E2-related factor 2 (Nrf2), the transcription factor regulating multiple antioxidant genes that contain the antioxidant response element (ARE) DNA sequence. This decline was accompanied by a decrease in protein and mRNA abundance of phase 2 antioxidant enzymes. For example, hepatic γ-glutamyl cysteine ligase catalytic unit (GCLC) mRNA abundance was decreased by ∼50% (P<0.0001) at Day +4 and further declined to ∼80% by Day +10 (P<0.0004) in Allo compared to Syn mice. Consistent with expectations, hepatic nuclear Nrf2 and TNF-α concentrations at Day +10 were inversely correlated (r2=0.8, p=0.01) in Allo mice (Figure 2 and 3). Together, these observations suggest that down-regulation of the Nrf2-GSH axis precedes stable elevation of TNF-α, and is an early predictor of the severity of GVHD in experimental transplantation. Future experiments will explore potential therapeutic implications of modulating the Nrf2 system in GVHD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.