Abstract
Abstract 4083
Pom is a third generation immunomodulatory drug (IMiD) which has demonstrated safety and efficacy in RRMM with prior exposure/resistant to other IMiDs and bortezomib. This is the first report on a phase II clinical trial employing Pomalidomide (Pom) in genomically defined high risk RRMM.
First cycle Pom was given at 4mg orally Days 1–21 every 28 days; dexamethasone (DEX) or bortezomib (BOZ) could be given in absence of PR or better from Cycle #3. In the absence of at least PR, Pom dose was escalated to 5mg. Cox regression modeling was employed for univariate and multivariate analyses, whereas Kaplan-Meier curves were used for overall survival (OS) and progression free survival (PFS).
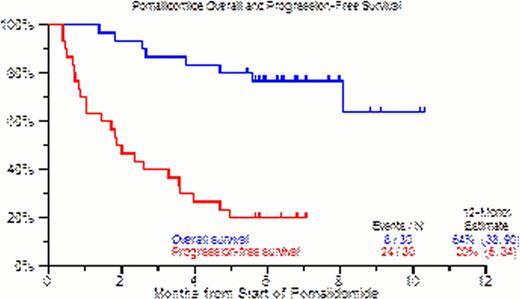
30 patients with RRMM were enrolled. Baseline characteristics included age >=65yr in 47%, ISS stage >=II was seen in 74% of patients, cytogenetic abnormalities (CA) within 6 months in 79%, and GEP-defined high risk in 66% by GEP70-gene model, 97% by GEP80-gene model, 13% had extramedullary disease (EMD), and 7% had secondary plasma cell leukemia (SPCL). 26/30 patients (87%) had prior autologous stem cell transplant, 23/30 patients (77%) had had at least 2 transplants. 22 patients (73%) had disease progression while on lenalidomide, bortezomib or both within 60 days of enrollment. At least 2 cycle of treatment were administered to 28 (93%) patients enrolled, 20 (67%) patients received >4 cycle of treatment and 16 (53%) received > 6 cycles. 15 patients (50%) discontinued therapy primarily due to progression or death. 5 (17%) patients achieved PR or better as best response. PFS benefit was observed in older patients (HR=0.29, p=0.008), with high LDH presenting an increased risk for OS (HR=14.64, p=0.013.) Most common toxicities, counting all toxicities (>=grade 3) were: thrombocytopenia (63%), leukopenia (63%), anemia (40%), hypophosphatemia (20%) and hypocalcemia (23%). Overall and progression- free survival at 12 months were 64% and 20%, respectively.
The present trial is the first investigation evaluating Pom's clinical activity in high risk RRMM and demonstrates good anti-myeloma activity in this heavily pre-treated patient population. Given the activity in poor prognostic clinical features such as EMD and SPCL, there is precedence to evaluate Pom as an IMiD of choice in combination with other novel agents for this subset of RRMM.
Drugs Administered by Cycle. Number of patients receiving given drug combinations in given cycle. (Note: the denominator used for calculating percentages is number of patients who started the cycle. In some columns, percentages do not add up to 100% because data was missing for some patients.)
| . | Cycle 1 (n=30) . | Cycle 2 (n=28) . | Cycle 3 (n=23) . | Cycle 4 (n=20) . | Cycle 5 (n=17) . | Cycle 6 (n=16) . |
|---|---|---|---|---|---|---|
| Pom alone | 29 (100%) | 28 (100%) | 12 (52%) | 7 (35%) | 5 (29%) | 4 (25%) |
| Pom + Dex | 0 (0%) | 0 (0%) | 9 (39%) | 5 (25%) | 5 (29%) | 4 (25%) |
| Pom + Vel | 0 (0%) | 0 (0%) | 0 (0%) | 1 (5%) | 2 (12%) | 2 (13%) |
| Pom + Dex + Vel | 0 (0%) | 0 (0%) | 1 (4%) | 6 (30%) | 4 (24%) | 3 (19%) |
| . | Cycle 1 (n=30) . | Cycle 2 (n=28) . | Cycle 3 (n=23) . | Cycle 4 (n=20) . | Cycle 5 (n=17) . | Cycle 6 (n=16) . |
|---|---|---|---|---|---|---|
| Pom alone | 29 (100%) | 28 (100%) | 12 (52%) | 7 (35%) | 5 (29%) | 4 (25%) |
| Pom + Dex | 0 (0%) | 0 (0%) | 9 (39%) | 5 (25%) | 5 (29%) | 4 (25%) |
| Pom + Vel | 0 (0%) | 0 (0%) | 0 (0%) | 1 (5%) | 2 (12%) | 2 (13%) |
| Pom + Dex + Vel | 0 (0%) | 0 (0%) | 1 (4%) | 6 (30%) | 4 (24%) | 3 (19%) |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.