Abstract
Abstract 405
Recently, monoallelic mutations in the zinc-finger transcription factor GATA2 have been shown to be responsible for GATA2-deficiency, a syndrome characterized by opportunistic infections, frequently atypical mycobacterial infections or MAC, and a hypocellular myelodysplastic syndrome (MDS) that transforms into acute myelogenous leukemia (AML). GATA2-deficiency was previously known by several other names: MonoMAC (Monocytopenia and Atypical Mycobacterial Infection), DCML (Dendritic Cell Monocyte, Lymphoid Deficiency), Familial MDS/AML, or Emberger syndrome (lymphedema with monosomy 7). Heterogeneous genetic defects in GATA2 result in haploinsufficiency in both spontaneous and familial forms of the disease. Predicting the transformation from MDS to AML in GATA2-deficiency has clinical implications for both prognosis and the timing of hematopoietic stem cell transplantation. ASXL1, a gene related to the Drosophilia additional sex combs gene, encodes a chromatin binding/transcription repressor protein that is frequently mutated in MDS/AML. Mutations in ASXL1 are associated with reduced time to progression to AML and poor overall survival, independent of IPSS score.
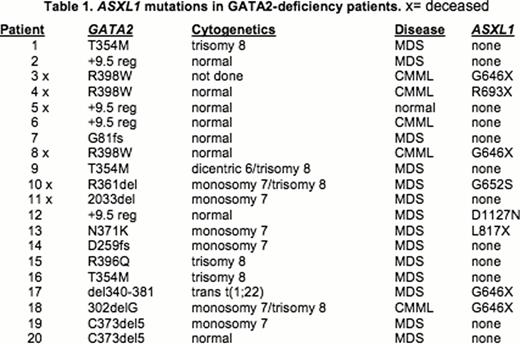
We sequenced the critical region of the ASXL1 gene in 20 patients with GATA2- deficiency to determine the frequency of ASXL1 mutations, and to correlate the presence of ASXL1 mutations with hematopoietic transformation. Since the ASXL1 mutations described in hematopoietic malignancies are located within the coding sequence of the two 3Õ-terminal exons (COSMIC: Catalogue of Somatic Mutations in Cancer), this ∼4.3kb region of ASLX1 was amplified by PCR and sequenced using five overlapping primer sets with substrate DNA isolated from mononuclear cell and granulocyte cell preparations from peripheral blood or bone marrow aspirates, or from extracts prepared from unfixed, unstained bone marrow aspirates. Mutations were confirmed with at least two independent PCR reactions with two unique primer sets.
Somatic ASXL1 mutations were detected in 8 of 20 patients with GATA2 mutations, 19/20 of whom had MDS. Five of these ASXL1 mutations have been previously associated with MDS/AML, including four independent cases of the most frequently described ASXL1 mutation (G646fs*12insG). The other four mutations were found once each; two of these were previously unreported (G652S(G>A) and L817fs*1delT). The patient cohort included two sisters with the same germline GATA2R398W mutation, but different somatic ASXL1 mutations (G464fs*12insG and R693X(C>T)). ASXL1 mutations were found in 4/5 GATA2- deficiency patients whose MDS had transformed into chronic myelomonocytic leukemia (CMML). Overall survival was lower for GATA2-deficiency patients with ASXL1 mutation (50% survival) compared to patients without ASXL1 mutation (83% survival), and was independent of IPSS score.
ASXL1 is frequently mutated in patients with GATA2-deficiency with at least 40% of patients having a mutation in ASXL1 compared to a 10–15% mutation rate reported for all MDS/AML patients. ASXL1 mutation correlates with the development of CMML and with poor overall survival, as reported previously for MDS/AML patients. There was no correlation between the presence and type of ASXL1 mutation and the specific GATA2 mutation: the eight different ASXL1 mutation events were found in six different GATA2 mutant backgrounds. These results are directly relevant to the prognosis and the timing of hematopoietic stem cell transplantation for GATA2-deficiency.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.