Abstract
Abstract  3907
3907
Cytogenetic abnormalities are among the most important predictors of clinical course and response to therapy in patients (pts) with chronic lymphocytic leukemia (CLL). Conventional chromosome banding (CBA) and fluorescence in situ hybridization (FISH) analyses detect abnormalities in 40–50% and 80% of pts, respectively. Trisomy 12 (+12), observed in ∼20% of CLL pts by FISH, is associated with atypical morphology and immunophenotype, and a more aggressive clinical course. We, therefore, review the clinical characteristics and outcome of 312 CLL pts with +12 evaluated at our center between 1988 and 2011.
FISH analysis for common abnormalities associated with CLL was performed on interphase nuclei obtained from cultured bone marrow cells using a multi-color probe panel designed to detect deletions of 11q22.3 (ATM), 13q14.3 (D13S319), 13q34 (LAMP1), 17p13.1 (TP53) and trisomy 12 (12p11.1-q11) (Abbott Molecular, Abbott Park, IL). Survival curves were calculated using Kaplan-Meier estimates and compared using the log-rank test. Differences were considered significant for p < 0.05.
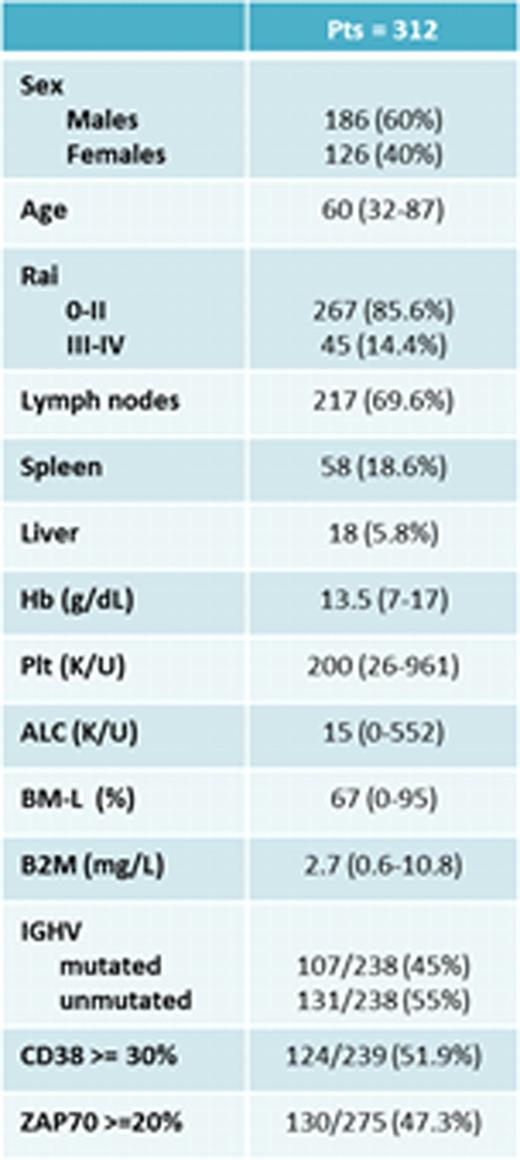
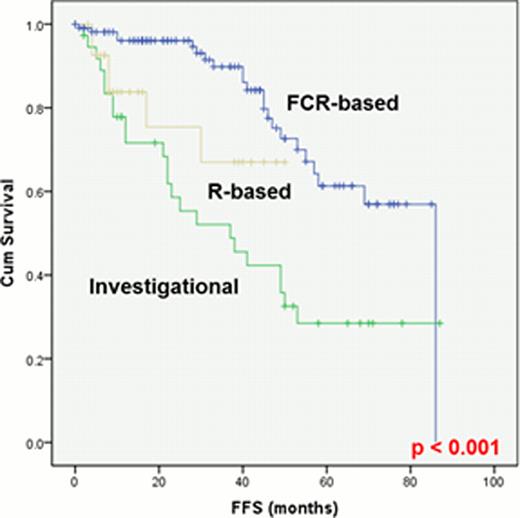
Patient characteristics at diagnosis are presented in Table 1. Of 215 pts assessed by both CBA and FISH, 105 were positive for +12 by both analyses and 110 were positive only by FISH. By CBA (112 pts, including 7 assessed only by CBA), +12 was the sole abnormality in 52 pts (47%); +12 was associated with +19 in 17 pts (16%), with del(14q) in 9 pts (8%), with +18 in 8 pts (7%), with +8 in 3 pts (3%), with del(13q) in 3 pts (3%), with t(14;19)(q32;q13) in 3 pts (3%) and with other abnormalities in 17 pts (13%). By FISH (287 pts), +12 was the sole abnormality in 225 pts (78%) and was associated with del(13q) in 62 pts (22%). The median number of interphase nuclei positive for +12 by FISH was 47% (range, 5–93%). One-hundred-eighty-seven pts (60%) needed treatment, with a median Time-To-Treatment (TTT) of 46 months (range, 35–56). The TTT was significantly shorter in pts with Rai stage III-IV disease, splenomegaly, lymphadenopathy, B2m > 4 mg/L, CD38+, ZAP70+, +12 detected by both CBA and FISH, and +12 associated with del(14q) or t(14;19). All 187 pts with progressive disease received treatment: 105 with an FCR-based regimen, 28 with rituximab(R)-based therapy (R+ GM-CSF or R+ methylprednisolone), and 28 with investigational drugs (Lenalidomide, R+ lenalidomide, GS101, or Ibrutinib). Overall response rate was 98%, 89% and 96%, respectively, whereas complete remission rate was 87%, 11% and 36%, respectively. Fifty-five pts failed first-line treatment; their median Failure-Free Survival (FFS) was 27 months (range, 0–87). The FFS was significantly longer in pts who received FCR-based regimens (p<0.001)(Fig 1). The median overall survival (OS) has not been reached, and only 33 pts have died. The OS was significantly shorter in pts older than 65 years, with ALC > 30,000, and with a median +12 positivity in >30% of interphase nuclei by FISH. A trend toward longer OS was observed for pts with +12 associated with +19 (p=0.07). Richter's Syndrome (RS) and second malignancies (SM) were the leading causes of death (5 and 13 of 33 deaths, respectively). RS was reported in 12 pts (4%), after a median time of 36 months from diagnosis. SM was reported in 31 pts (10%), after a median time of 30 months from diagnosis. At the time of diagnosis of SM, 13 patients had received a therapy for CLL and 18 were untreated.
In conclusion, pts with CLL and +12 have unique laboratory and clinical features. A high proportion develops progressive disease and requires treatment. Among available therapies, FCR-based regimens are associated with a longer FFS. A high rate of SM is observed in pts with +12, including in pts who have not received prior treatment
Wierda:Abbott Laboratories: Research Funding. O'Brien:Avila: Research Funding; Bayer: Consultancy; Bristol-Myers Squibb: Research Funding; Gilead Sciences: Consultancy, Research Funding; Celgene: Consultancy; Cephalon: Consultancy; CII Global Research Foundation: Membership on an entity's Board of Directors or advisory committees; Genentech BioOncology: Research Funding; Genzyme: Consultancy; GlaxoSmithKline: Consultancy; MorphoSys: Consultancy; Novartis: Consultancy; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy; Seattle Genetics, Inc.: Consultancy; Sigma Tau Pharmaceuticals: Consultancy; Talon: Research Funding; The Medal Group: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract