Abstract
Abstract 3901
Angiogenesis is elevated in many hematological malignancies, but there is limited information for angiogenesis in Waldenstrom's macroglobulinemia (WM). Several cytokines including VEGF (and its major angiogenic component VEGF-A), bFGF, angiogenin and angiopoietin-1 (Ang-1) and 2 (Ang-2) participate in the neoangiogenesis process. We have previously shown that circulating angiogenic cytokines correlate with disease severity in WM (Anagnostopoulos et al, Br J Haematol 2007;137:560–8), while it has been reported that the bone marrow (BM) microvessel density (MVD) is increased in 30%-40% of patients with WM (Rajkumar et al, Semin Oncol 2003;30:265–9). The ratio of Ang-1/Ang-2 correlates with survival in multiple myeloma, but there is no information for the prognostic value of the angiopoietins and other angiogenic cytokines in WM.
To address this issue, we studied the serum levels of VEGF, VEGF-A, bFGF, angiogenin, Ang-1 and Ang-2 in the serum of 55 patients with symptomatic WM before the administration of any kind of therapy, in 5 patients with asymptomatic WM (AWM), in 12 patients with IgM MGUS and in 30 healthy controls, of similar age and gender. Circulating VEGF, VEGF-A, bFGF, angiogenin, Ang-1 and Ang-2 were measured using ELISA method (R&D Systems, Minneapolis, MN, USA for VEGF, bFGF, angiogenin, Ang-1 and, Ang-2; Diaclone SAS, Besancon, France for VEGF-A). MVD was also evaluated in the BM biopsies of all patients, according to standard methodology. Table 1 depicts the levels of the studied angiogenic cytokines. The serum levels of VEGF, VEGFA, bFGF, angiogenin and Ang-2 were markedly elevated in WM patients compared to controls (p<0.001 for all comparisons); Ang-1 levels (p<0.01) were lower in WM patients than in controls, and the corresponding Ang-1 to Ang-2 ratio was significantly lower in WM patients than in controls, further indicating an angiogenic shift in WM patients. Circulating angiogenin (p<0.001) and Ang-2 (p=0.001) levels were also increased in WM patients compared to patients with IgM MGUS but Ang-1 levels were lower (p=0.003) resulting in Ang 1/2 ratio significantly higher in IgM MGUS than WM patients (p=0.004). In symptomatic WM patients, Ang-2 levels and the corresponding Ang-1/2 ratio correlated with serum beta2-microglobulin (r=0.454, p=0.002 and r=0.459, p=0.003, respectively). Ang-2 levels correlated with ISSWM stage (1914 ± 1175 pg/ml vs. 4461 ± 2628 pg/ml vs. 3926 ± 2172 pg/ml, for ISSWM-low, intermediate and high risk, respectively, p=0.007). A strong inverse correlation of MVD to Ang-1 was found in patients with WM (r=-0.600, p=0.011), indicative of the regulatory function of Ang-1 as an antagonist of vessel sprouting and new vessel formation.
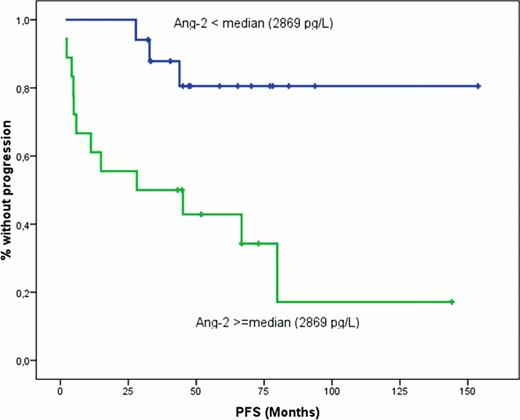
The median follow-up of symptomatic WM patients was 35 months. The median overall survival (OS) has not been reached yet, while the probability for 3-year OS was 76%. The median progression-free survival (PFS) was 57 months and the 3-year probability of PFS was 56%. Patients with Ang-2 levels above the median value had significantly shorter PFS (3-year PFS rate 82% vs. 50%, p=0.032; Figure).
We conclude that in patients with WM, serum levels of several angiogenic cytokines (Ang-2, angiogenin, VEGF, VEGF-A, and bFGF) are markedly elevated and for some of these cytokines there is a correlation with disease features. The levels of pro-angiogenic cytokines, such as Ang-2 increase as disease evolves for IgM MGUS to symptomatic WM, while the Ang-1 decreases. We show that the MVD in the BM strongly correlates to decreasing Ang-1 levels, probably due to the decreasing inhibitory effect of Ang-1 to vessel formation in the BM microenvironment as disease evolves. For the first time we found that Ang-2, may also have a prognostic significance, associated with significantly shorter PFS. Further follow up is needed in order to evaluate the prognostic significance of Ang-2 for the OS of patients with WM
Levels of the studied circulating angiogenic cytokines (mean ± SD)
| . | Controls . | IgM MGUS . | aWM . | WM . |
|---|---|---|---|---|
| VEGF (pg/ml) | 106 ± 76 | 323 ± 217 | 116 ± 83 | 399 ± 248 |
| VEGF-A (pg/ml) | 6.7 ± 13.6 | 97.5 ± 94 | 28.4 ± 49.3 | 113 ± 113 |
| bFGF (pg/ml) | 1.3 ± 3.2 | 12 ± 14 | 9.5 ± 16 | 17 ± 21.6 |
| Angiogenin (pg/ml) | 2.4×105 ± 0.5×105 | 2.7×105 ± 0.8×105 | 2.8×105 ± 0.7×105 | 4.5×105 ± 2.1×105 |
| Ang-1 (pg/ml) | 4.8×105 ± 1.1×105 | 4.8×105 ± 1.9×105 | 2.6×105 ± 1.5×105 | 2.5×105 ± 2.3×105 |
| Ang-2 (pg/ml) | 1747 ± 1023 | 1783 ± 684 | 3775 ± 1296 | 3424 ± 2570 |
| Ang-1/2 ratio | 28.1 ± 11.6 | 31 ± 14 | 6.5 ± 1.6 | 14.5 ± 17.7 |
| . | Controls . | IgM MGUS . | aWM . | WM . |
|---|---|---|---|---|
| VEGF (pg/ml) | 106 ± 76 | 323 ± 217 | 116 ± 83 | 399 ± 248 |
| VEGF-A (pg/ml) | 6.7 ± 13.6 | 97.5 ± 94 | 28.4 ± 49.3 | 113 ± 113 |
| bFGF (pg/ml) | 1.3 ± 3.2 | 12 ± 14 | 9.5 ± 16 | 17 ± 21.6 |
| Angiogenin (pg/ml) | 2.4×105 ± 0.5×105 | 2.7×105 ± 0.8×105 | 2.8×105 ± 0.7×105 | 4.5×105 ± 2.1×105 |
| Ang-1 (pg/ml) | 4.8×105 ± 1.1×105 | 4.8×105 ± 1.9×105 | 2.6×105 ± 1.5×105 | 2.5×105 ± 2.3×105 |
| Ang-2 (pg/ml) | 1747 ± 1023 | 1783 ± 684 | 3775 ± 1296 | 3424 ± 2570 |
| Ang-1/2 ratio | 28.1 ± 11.6 | 31 ± 14 | 6.5 ± 1.6 | 14.5 ± 17.7 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.