Abstract
Abstract 2902
Although chronic lymphocytic leukemia (CLL) is a low grade B-cell malignancy, 2–8% of patients (pts) are reported to undergo transformation to diffuse large B-cell lymphoma (DLBCL; Richter's syndrome [RS]). Whether the risk of transformation is related to the underlying biology of CLL or is a consequence of treatment for CLL is uncertain. Also, the role of prognostic markers such as immunoglobulin heavy chain (IGHV) gene mutation status, ZAP-70 and CD38 expression, and genetic abnormalities detected by fluorescence in situ hybridization (FISH) in predicting the development of RS is not well defined. The available data on stem cell transplant (SCT) in pts with RS is based on retrospective case series of selected RS pts and is of uncertain relevance to the majority of CLL pts experiencing RS. Therefore, we conducted a cohort study of newly diagnosed CLL pts to explore the incidence and natural history of RS.
The Mayo Clinic CLL database includes all consenting pts with a pathologic diagnosis of CLL. Information on date of diagnosis, treatment history, and disease-related complications is abstracted from clinical records on all pts and then maintained prospectively. We used this database to identify newly diagnosed pts with CLL from January 2000 through July 2011, who were seen at our institution within 12 months of diagnosis. Pts with biopsy-confirmed RS were included in this analysis. For pts referred from an outside institution, pathology review at Mayo Clinic was mandatory to be included in this study. The Mayo Clinic Institutional Review Board approved the study.
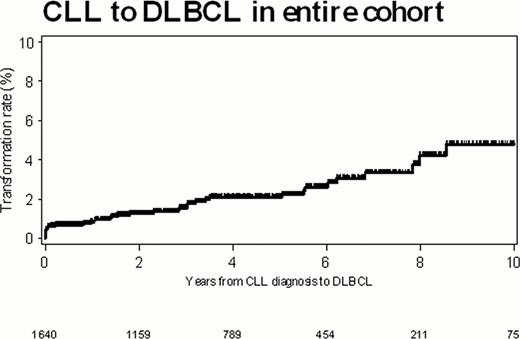
Between January 2000 and July 2011, 1641 pts with newly diagnosed CLL were seen at Mayo Clinic within 12 months of diagnosis, and this cohort had a median follow-up of 4.0 yrs (range, 0–12.3 yrs). Of these pts, 37 (2.3%) were diagnosed with RS. The median time to development of RS after a diagnosis of CLL was 1.8 yrs (range, 0–11.7 yrs). The incidence of RS at 2 and 5 yrs post CLL diagnosis were 1.3% and 2.1% respectively (Figure).
Pts who developed RS had more advanced (Rai III/IV) stage disease (22% vs. 6%; p<0.001) and higher beta 2-microglobulin levels (median 3.1 mg/dL vs. 2.3 mg/dL; p=0.003) at diagnosis of their CLL compared to pts without RS. Pts who developed RS were also more likely to be positive for ZAP-70 (58% vs. 33%; p=0.03), CD49d (77% vs. 31%; p=0.0004), and CD38 (57% vs. 29%; p=0.001), have unmutated IGHV (76% vs. 44%; p=0.003) and have either del(11q22.3) or del(17p13) on FISH analysis (46% vs. 13%; p<0.0001).
RS transformation occurred prior to treatment of CLL in 17 (46%) pts and after treatment of CLL in 20 pts (54%). Treatment for CLL was associated with an increased risk of RS (92% vs 34%; p<0.0001). The odds ratio (OR) of RS was increased for pts who received purine nucleoside analogues (OR=3.0; 95%CI=[1.6, 5.9]; p=0.001), alkylating agents (OR=2.5; 95% CI=[1.3, 4.9]; p=0.004), or both (OR=3.3; 95% CI=[1.7, 6.4]; p=0.0003) for treatment of their CLL.
Median overall survival after development of RS was 2.1 yrs. Most pts (29/37, 78%) received R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) therapy. Two (5%) pts underwent an autologous SCT and 5 (14%) underwent an allogeneic SCT. Additionally, 1 (3%) patient received an autologous SCT followed by an allogeneic SCT. By the end of the study period, 17 (46%) pts with RS had died.
The overall rate of biopsy-proven RS in this large cohort of pts with newly diagnosed CLL was 2.3% (5 year incidence: 2.1%). Clinical characteristics (Rai stage III/IV) and biologic characteristics of the CLL B-cell (positive expression of ZAP-70, CD38 and CD49d; unmutated IGHV; and either del(11q22.3) or del(17p13) on FISH) at the time of diagnosis were associated with future risk of RS. Exposure to purine nucleoside analogues and/or alkylating agents for treatment of CLL was also associated with increased risk of RS.
Zent:GlaxoSmithKline: Research Funding; Novartis: Research Funding; Genentech: Research Funding; Genzyme: Research Funding; Biothera: Research Funding. Shanafelt:Genentech: Research Funding; Glaxo-Smith-Kline: Research Funding; Cephalon: Research Funding; Hospira: Research Funding; Celgene: Research Funding; Polyphenon E International: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.