Abstract
Abstract 2801
Recommendations for use of erythropoiesis-stimulating agents (ESAs) in anemic patients with MDS are based on baseline endogenous erythropoietin levels and red blood cell transfusion requirements, factors which predict the likelihood of a response to ESA treatment. These recommendations for ESA use have been incorporated into quality-of-care treatment guidelines for MDS. We examined whether baseline endogenous thrombopoietin (TPO) levels and platelet transfusion requirements likewise predict response of thrombocytopenic MDS patients to treatment with romiplostim, a TPO receptor agonist.
In a placebo(PBO)-controlled trial of romiplostim (randomized 2:1) in 250 thrombocytopenic [median (Q1, Q3) baseline platelet count 19.3 (12.5, 30.3) × 109/L] IPSS low/int-1 MDS patients, study drug was discontinued early due to data monitoring committee concerns that the potential small benefit seen in the reduction of bleeding did not outweigh the potential risk for disease progression to AML and that the transient increases in blast cell counts may put patients at risk for diagnosis of and treatment for AML. Hematologic improvement of platelets (HI-P, per IWG 2006) is defined as 8 consecutive weeks of an absolute platelet increase of 30×109/L (for patients with baseline platelet counts >20×109/L) or an increase from <20×109/L to >20×109/L and by at least 100% (for patients with baseline platelet counts <20×109/L). In this trial of romiplostim in MDS, HI-P rates were higher with romiplostim than PBO (36.5% vs. 3.6%, odds ratio 15.6, p<0.001) as were median platelet counts from Week 4 on (p<0.001). Data from this trial were used to examine the relationship between baseline TPO levels and platelet transfusion needs and outcomes. TPO levels (in pg/mL) were assessed by ELISA at baseline, weeks 14, 28, 42, and at the end of treatment. In this study, platelet response is defined as meeting the same criteria as HI-P, but for 1 week as opposed to for 8 consecutive weeks. As with the ESA model (Hellstrom-Lindberg BJH 1997), a TPO model was developed from log-likelihood ratios and logistic coefficients, with scaling of the log-likelihood ratios to obtain predictive scores. The TPO model was then validated using data from a previous phase 1/2 study of romiplostim in lower-risk thrombocytopenic MDS patients (Sekeres Cancer 2010, Kantarjian J Clin Onc 2009). Variables analyzed in formulating the model included baseline platelet count, number of platelet units transfused in the past year, and baseline endogenous TPO levels.
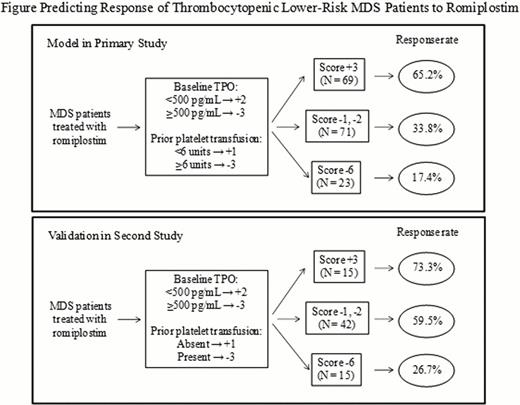
For romiplostim-treated patients (N = 167), the median age was 71 years, the most common WHO subgroups were RCMD (68.3%), RAEB-1 (14.4%), and MDS-U (9.6%), and IPSS scores were 0 (24.0%), 0.5 (51.5%), 1 (20.4%), 1.5 (0.6%), and missing (3.6%). Median (Q1, Q3) baseline TPO levels were 212 (84, 2290) pg/mL. Among romiplostim-treated patients, patients with an HI-P (vs. those not having an HI-P) had lower median baseline TPO levels (172 vs. 236 pg/mL, p = 0.3589) and lower mean baseline TPO levels (854 vs. 1,210, p = 0.0497), and were less likely to have had ≥6 platelet units transfused in the past year (p = 0.0027). For those with a platelet response during ≥50% of study weeks, median baseline TPO levels were lower (138 vs. 1,034 pg/mL, p = 0.0215) as were mean baseline TPO levels (695 vs. 1,390, p = 0.001) and the likelihood of having had ≥6 platelet units transfused in the past year (p = 0.0037). A model for predicting response to romiplostim (i.e., platelet response for ≥50% of weeks) in patients with lower-risk MDS was developed (Figure, top panel). Of note, history of prior platelet transfusion (<6 vs. ≥6 units in the past year) was a better predictor of platelet response than baseline platelet counts. The model was then validated in a second independent romiplostim monotherapy study in MDS, showing a similar pattern of response rates associated with baseline TPO levels and the presence of past platelet transfusions (Figure, bottom panel).
For thrombocytopenic patients with lower-risk MDS, lower baseline TPO levels (<500 pg/mL) and limited platelet transfusion history (<6 units in the past year) predict a greater likelihood that a patient will have a platelet response when treated with romiplostim.
Sekeres:Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees. Off Label Use: The use of romiplostim in MDS was examined in this abstract. Giagounidis:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Kantarjian:Amgen: Research Funding. Mufti:Celgene: Consultancy, Research Funding. Fenaux:Janssen: Honoraria, Research Funding; Roche: Honoraria, Research Funding; GlaxoSmithKline: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Amgen: Honoraria, Research Funding. Jia:Amgen: Employment, Equity Ownership. Yang:Amgen: Employment, Equity Ownership. Platzbecker:Novartis: Consultancy; Celgene: Consultancy; GlaxoSmithKline: Consultancy; Amgen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.