Abstract
Abstract 219
Prolonged intervals of thrombocytopenia are common after stem cell transplantation, and platelet transfusions are the only temporary therapy before engraftment. Risk of thrombocytopenia is greater in patients receiving total body irradiation (TBI) in their conditioning regimen. Eltrombopag is a small-molecule, nonpeptide oral agent that functions as an agonist of the thrombopoietin receptor. It is approved by the FDA for the treatment of chronic idiopathic thrombocytopenic purpura and is being developed as a treatment for thrombocytopenia of other various etiologies. We report updated results of a Phase I clinical trial assessing the safety, pharmacokinetics (PK) and maximum tolerated dose (MTD) of once daily oral eltrombopag in patients undergoing stem cell transplantation with a conditioning regimen containing TBI ≥ 400 cGy.
A Phase I dose-escalation clinical trial was conducted to evaluate the safety and PK of eltrombopag at 4 different dose levels: 75, 150, 225, and 300 mg given once daily for 27 days, starting 24–48 hours post stem cell transplantation to eligible patients ≥18 years old. Patients with various indications for stem cell transplantation, KPS ≥70%, and TBI ≥ 400 cGy were eligible. Patients receiving either an autologous (Auto) or allogeneic (Allo) stem cell transplantation from a sibling, related donor, or matched unrelated donor (MUD) were eligible. Stem cells from peripheral blood (PBSC) or bone marrow were permitted; however, cord blood stem cell transplantation was not permitted. Patients at risk of thromboembolism or with a history of thromboembolic disease in the preceding 6 months were excluded. PK sampling was obtained over a 24 hour period after the first dose of eltrombopag as well as during the second week of treatment (steady-state).
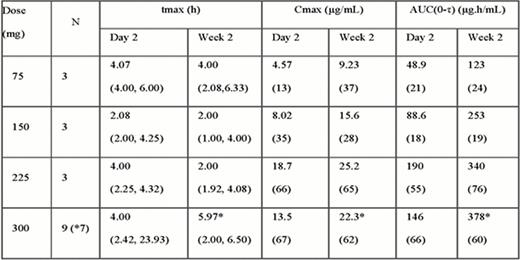
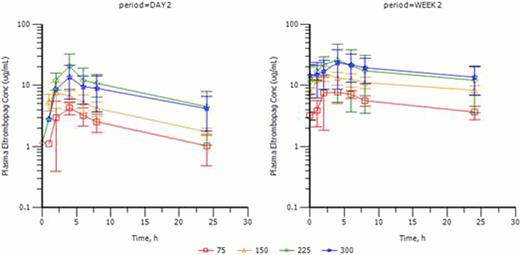
As of August 1 2012, a total of 19 subjects (7 AML, 4 lymphoma, 1 CML, 1 MDS, 1 myelofibrosis, 2 ALL, 1 APML, 1 Biphenotypic Leukemia and 1 CLL/SLL) were enrolled, and 15 completed protocol treatments. All 19 were PBSC transplants with 12 MUD, 6 Allo and 1 Auto. Three subjects were completed at each dose level up to 225 mg with six completing treatment at the highest dose of 300mg. Four subjects were replaced because drug compliance was less than 75%. To date, 11/19 are alive while 8/19 have died (3 related to graft vs. host disease (GVHD), 3 Infection-related and 2 related to disease progression (f/u interval: 5.7 – 30 months). No dose limiting toxicities (DLTs) have been observed. The most common adverse events (AEs) up to the 300 mg dose level were related to standard stem cell transplantation, which included low blood counts, fatigue, headache, diarrhea, nausea, peripheral edema, hypoalbuminemia, hyperglycemia, hypocalcemia, and hypomagnesemia. Possibly drug-related AEs were dry skin, rash, elevated creatinine, and hypokalemia; these were all grade 3 or less. There were 12 severe AEs observed in 8 subjects, which included infection (3/19), pulmonary embolism (PE) (1/19), acute renal failure (3/19), gastrointestinal (2/19), acute respiratory distress syndrome (1/19), pericarditis (1/19) and GvHD skin rash (1/19). Most SAEs were considered related to stem cell transplant, except one subject at 75 mg with PE possibly related to eltrombopag, but also possibly related to stem cell transplant and cancer diagnosis. The PE occurred 9 days after stopping eltrombopag at which time platelet count was 252K. Time to platelet engraftment and number of platelet transfusions were also documented for each enrolled patient. Median time to platelet engraftment for all subjects on this study was 16 days. PK data are summarized in Table 1 and Figure 1. A dose-dependent increase in plasma exposure of eltrombopag was observed; although some saturation of absorption is evident at the 300 mg dose level. (Figure 1).
27-day once daily dosing of eltrombopag to enhance platelet recovery for post-transplant thrombocytopenia is well tolerated, with no DLTs observed up to the 300 mg dose level. Most AEs were transplant related. PK showed proportional plasma concentration up to 225 mg daily dosing, with some saturation of drug absorption above 225 mg.
Mean (± SD) plasma eltrombopag concentration time profiles for 75, 150, 225, and 300 mg dose levels
Mean (± SD) plasma eltrombopag concentration time profiles for 75, 150, 225, and 300 mg dose levels
Liesveld:Eisai: Research Funding, Speakers Bureau; Bristol-Myers Squibb: Speakers Bureau; Ariad: Honoraria. Off Label Use: Eltrombopag not approved post-transplant. Dawson:GlaxoSmithKline: Employment.
Author notes
Asterisk with author names denotes non-ASH members.