Abstract
Abstract 200
In a multicenter phase 3 randomized trial, VMPT-VT was superior to VMP for response rates, progression-free survival and time to next treatment (Palumbo A, et al. J Clin Oncol 2010). Here we report an updated analysis on survival after 4 years of follow-up.
Patients (N=511) were randomly assigned to receive nine 6-week cycles of VMPT-VT (induction: bortezomib 1.3 mg/m2, d 1, 4, 8, 11, 22, 25, 29, 32, cycles 1–4, d 1, 8, 22, 29, cycles 5–9; melphalan 9 mg/m2 d 1–4, prednisone 60 mg/m2, d 1–4, thalidomide 50 mg d 1–42; maintenance: bortezomib 1.3 mg/m2 every 14 days and thalidomide 50 mg/day up to 2 years) or VMP alone. After the inclusion of 139 patients, the protocol was amended: both VMPT-VT and VMP induction schedules were changed to nine 5-week cycles and bortezomib schedule was modified to weekly administration (1.3 mg/m2 d 1,8,15,22, all cycles).
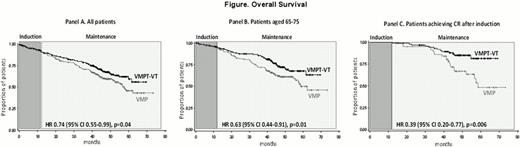
After a median follow-up of 47.2 months, median OS was not reached in the VMPT-VT arm and was 58.2 months in the VMP arm; 5-year OS rates were 59.3% and 45.9%, respectively (HR 0.74, p=0.04), with 26% reduced risk of death for patients receiving VMPT-VT (Figure-panel A). This benefit was more evident in patients younger than 75 years (5-year rates 67.8% for VMPT-VT vs 49.9% for VMP, HR 0.63, p=0.01, Figure-panel B) and in patients in complete response (CR) after induction (5-year rates 81.4% for VMPT-VT vs 48.2% for VMP, HR 0.38, p=0.006, Figure-panel C) while no significant differences were evident in patients with standard- or high-risk features detected by FISH (HR 0.99, p=0.99). A 1-year landmark analysis for patients completing induction was performed: the 4-year OS was 64.6% in the VMPT-VT group and 49.7% in the VMP group, with 33% reduced the risk of death for patients receiving VT maintenance (HR 0.67, p=0.02). Forty-nine percent of VMPT-VT and 70% of VMP patients relapsed and received subsequent salvage therapies; there was no difference in survival from relapse in the two groups (2-year OS rates 40.7% vs 50.2%,HR 1.11, p=0.54).
The median duration of VT maintenance was 23.8 months. During VT maintenance 7% of patients experienced grade 3–4 peripheral neuropathy, 5% grade 3–4 hematological toxicity, 3% grade 3–4 infection and 12% discontinued due to adverse events. Second primary malignancies were reported in 7/254 patients in the VMPT-VT group and 7/257 patients in the VMP group. These corresponded to incidence rates of 0.9 and 1.05 per 100 patient-years, respectively, and were consistent with background incidence rates in the general population (aged 65–74 years 1.9, aged ≥ 75 years 2.3, SEER database).
VMPT-VT significantly prolonged OS compared with VMP, especially in patients younger than 75 years and in patients achieving CR after induction. In patients 67–75 years of age, VMPT-VT reduced the risk of death by 37% and it should be considered a new standard of care.
Palumbo:Celgene: Advisory Board, Advisory Board Other, Consultancy, Honoraria; Janssen: Advisory Board Other, Consultancy, Honoraria. Bringhen:Janssen: Honoraria; Celgene: Honoraria. Gentilini:Janssen: Honoraria; Celgene: Honoraria. Patriarca:Janssen: Honoraria. Guglielmelli:Janssen: Honoraria; Celgene: Honoraria. Musto:Janssen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Petrucci:Janssen: Honoraria; Celgene: Honoraria. Boccadoro:Janssen: Consultancy, Research Funding, Scientific Advisory Board Other; Celgene: Consultancy, Research Funding, Scientific Advisory Board, Scientific Advisory Board Other.
Author notes
Asterisk with author names denotes non-ASH members.