Abstract
von Willebrand factor (VWF) is a promising target for developing antithrombotic drugs. The absence of accessible animal models impedes the study of specific human VWF (huVWF) targeting molecules in thrombosis. huVWF is not functional in the mouse because of a lack of interaction between huVWF and murine glycoprotein Ib. Using site-directed mutagenesis, we have replaced single or multiple amino acids in huVWF with their murine counterparts to eliminate species incompatibility. Using hydrodynamic injection, we have expressed the different chimeric VWF constructs into VWF−/− mice. Only huVWF with a complete murine A1 domain insertion was able to correct bleeding in vivo and form occlusive thrombi in mesenteric vessels after FeCl3 treatment. Using this model, we tested the antithrombotic effect of monoclonal antibodies against huVWF, blocking its interaction with collagens (mAbs 203 and 505) or with glycoprotein IIbIIIa (mAb 9). The 3 mAbs inhibited the thrombotic process in arterioles of VWF−/− mice expressing huVWFmuA1. Inhibiting VWF-interaction with collagens was more potent, emphasizing the potential of such a target as an antithrombotic tool. Our results validate our murine model as a simple in vivo tool to evaluate anti-huVWF agents.
Introduction
Cardiovascular and cerebrovascular diseases represent one of the leading causes of morbidity and mortality in industrialized countries.1 At the center of these diseases, the blood platelet is of major importance.2 Indeed, excessive accumulation of platelets at sites of atherosclerotic plaque rupture is the crucial pathogenic event that is responsible for the development of the acute coronary syndrome, stroke, and the ischemic complications of peripheral vascular disease.3 The importance of platelets is reflected in the increasing number of patients receiving antiplatelet therapy, such as aspirin, clopidogrel, and anti-glycoprotein (GP) IIbIIIa inhibitors.4 In search for new target molecules, several lines of evidence point to antiadhesive drugs. Indeed, interfering with the first step in platelet adhesion to the subendothelium seems like a viable option, and the multimeric plasma protein VWF is at the center of this essential adhesion step. Indeed, when the endothelial layer is disrupted (after vascular injury or plaque rupture), fibrillar collagens present in the subendothelium are exposed to the flowing blood. VWF binds to collagen through its A3 domain and adopts a conformation that allows binding of its A1 domain to GPIb on platelets.5,6 This interaction is of particular relevance at high shear rates where the VWF-GPIb interaction is the only one able to resist to rheologic conditions existing in stenosed arteries. At low shear, platelet-collagen interactions can occur directly and involve 2 collagen receptors on platelets, α2β1 and GPVI. The VWF-GPIb interaction is transient and results in rolling and slowing down of platelets. During this rolling phase, inside-out signaling leads to activation of GPIIbIIIa and subsequent platelet aggregation through binding of GPIIbIIIa to fibrinogen and/or VWF. Thus, inhibition of the VWF-GpIb axis would target the very early phase of platelet adhesion. Antibodies directed against VWF or GPIb have been shown to have potent antithrombotic activity in vivo in nonhuman primates and induce less bleeding risks than anti-GPIIbIIIa.7 Nonantibody strategies targeting the VWF-GPIb axis involve aptamers, such as the ARC1779, for which initial clinical trials appear promising.8,9 Similarly, antibodies targeting the VWF-collagen interaction have also been tested, and abolition of arterial thrombus formation was observed in baboons.10 Altogether, these results emphasize the critical role of VWF in the thrombotic response.11 Our laboratory has developed a range of anti–human VWF monoclonal antibodies (mAb) targeting specifically each VWF interaction involved in thrombus formation (ie, interaction with GPIb, with collagens, and with GPIIbIIIa).12-14 Despite their extensive in vitro characterization, the antithrombotic potential of these antibodies was never tested in vivo in some easily accessible animal models, such as the mouse, because of species incompatibility. Indeed, these mAbs are directed against human VWF and do not cross-react with mouse Vwf. In addition, human VWF could not be injected in the mouse because it does not interact with mouse platelet GPIb. Our goal in the present study was therefore to develop a model in which our antibodies as well as other drugs targeting human VWF could be tested rapidly in mouse models of thrombosis. By engineering a human-murine chimeric VWF molecule that we expressed in the VWF-deficient mouse, we were able to evaluate the antithrombotic potential of antibodies inhibiting VWF binding to collagens and to GPIIbIIIa.
Methods
Selection and construction of huVWF mutants
Animal experiments received the approval of the French Veterinary Services under the authority of the French Minister of Agriculture. Our official agreement number is B 94–043-13. Generation of the expression vectors pcDNA6-muVwf and pLIVE-muVwf encoding for full-length murine Vwf (muVwf) has been previously described.15,16 The pCDNA6-huVWF plasmid containing the full-length cDNA of human VWF (huVWF) was obtained as previously described.17 This plasmid was used as a template for the generation of 7 different cDNAs constructs of human VWF with site-directed mutagenesis by overlap extension using the PCR.18 In 2 constructs, a single amino acid was modified: huVWF H1326R and huVWF Q1391P. The sequences of the mutated primers used for their generation are shown in Table 1. Five additional constructs were engineered with increasing murine participation until the entire A1 domain of huVWF was replaced by the murine A1 domain (residues 1260-1479). These constructs were obtained using a PCR-based method using partially overlapping primers consisting of hybrid oligonucleotides (sequence shown in Table 1) of huVWF and muVwf cDNAs.19 In 3 constructs, an exposed loop of huVWF amino acids was substituted by the corresponding muVwf sequence: huVWFmu (1306-1314), huVWFmu (1326-1333), and huVWFmu (1370-1385). In the fourth construct, a combination of 2 exposed loops of amino acids were modified: huVWFmu (1326-1333; 1370-1385). In the fifth construct, huVWFmuA1, the entire A1 domain of huVWF was replaced by the murine A1 domain (residues 1260-1479). Each cDNA construct was confirmed by DNA analysis using the ABI PRISM Dye Terminator Cycle Sequencing Reaction Kit Version 3.1 (Applied Biosystems) on an ABI PRISM 310 DNA sequencer according to the manufacturer's specifications. After sequence analysis, all the constructs were cloned in pCDNA6, then amplified in Escherichia coli DH5α cells and purified by a Nucleobond endotoxin-free plasmid DNA PC 2000 kit (Macherey-Nagel) according to the manufacturer's instructions. The purity and quantity of the plasmids DNA were analyzed by agarose gel electrophoresis and by absorbance at 260 and 280 nm. Finally, an original sequence of huVWF, containing the mouse A1 sequence, was designed and introduced in pLIVE by GeneArt (Geneart/Life Technologies) respecting the general cloning architecture promoting the optimal translation efficiency of the VWF mRNA.
Mice
Four- to 8-week-old VWF-deficient mice (VWF−/− mice)20 on a C57Bl/6 background, bred in the Inserm U770 animal facility, were used throughout this study. Housing and experiments were done as recommended by French regulations and the experimental guidelines of the European Community.
Hydrodynamic injection
A total of 150 μg of plasmid DNA (pLIVE or pCDNA6) containing the different constructs diluted in a volume of saline (0.9% NaCl) equivalent to 10% of the mouse body weight were injected into the tail vein within 5 seconds as described.15 A 2-mL syringe with a 26.5-gauge needle was used.
Blood collection
Mice were anesthetized by intraperitoneal injection of tribromoethanol (0.15 mL per 10 g of body weight), and blood was collected via retro-orbital puncture in trisodium citrate (9 volumes of blood to 1 volume of 0.138M trisodium citrate). To obtain platelet-poor plasma, blood samples were centrifuged at 1000g for 20 minutes at 22°C. Plasma was kept at −80°C until use. For Bioflux experiments, blood was collected by cardiac puncture in trisodium citrate in presence of 80μM of PPACK.
Determination of VWF levels by ELISA
Plasma was collected 24 hours or 4 days after hydrodynamic injection according to the vector that was used (pCDNA6 or pLIVE, respectively), unless indicated otherwise.15,16 Plasma VWF was quantified by ELISA using a polyclonal antibody anti-huVWF (Dako France) and horseradish peroxidase–conjugated polyclonal antibody anti-huVWF (Dako France).21 Normal pool plasma from 20 C57BL/6J mice (Janvier) and normal pool plasma from healthy human donors (Cryopep) were used as reference and set at 100% Results were expressed as percentage of normal VWF levels.
Determination of endogenous murine ADAMTS13 levels
Blood was collected in lithium-heparin tubes before, 1 or 4 days after hydrodynamic injection. After preparation of plasma, samples were diluted 6.7-, 10-, and 20-fold in 50 μL 0.005% Tween-20, 25mM CaCl2, 5mM BisTris (pH 6.0), and 50 μL of FRETS-VWF73 substrate (diluted 1:25 in the same buffer; Peptanova GmbH) was added. Fluorescence (excitation at 340 nm, emission at 440 nm) was monitored for 60 minutes at a 5-minute interval at 30°C. Pooled plasma of noninjected mice was used as control. Results are presented as percentage of normal ADAMTS levels.
VWF multimeric structure analysis
The multimeric structure of VWF was analyzed by 0.1% SDS and 2% agarose (Seakemr HGT Agarose; Lonza Walkersville) gel electrophoresis as described.22 Multimers were visualized using an alkaline phosphatase-congugated anti–human VWF polyclonal antibody.
Bleeding time
Mouse tail bleeding was performed as described.16 Briefly, 3 mm of distal tail was cut using a scalpel. The amputated tail was immersed immediately in physiologic saline at 37°C, and bleeding time was measured from the moment of the transection until the first arrest of bleeding. Observation was stopped at 600 seconds when bleeding did not cease.
Thrombus formation under flow
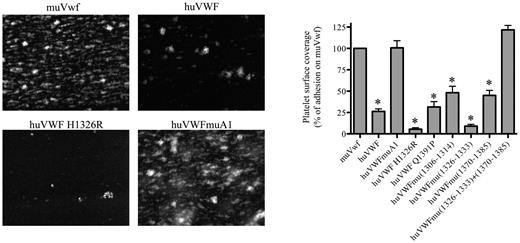
Blood perfusion experiments were performed in a parallel plate perfusion chamber essentially as described previously.23 PPACK (80μM) anticoagulated blood from VWF-expressing mice was incubated with rhodamine 6G (10 μg/mL) for 5 minutes at 37°C and then perfused on glass coverslips precoated overnight at 4°C with fibrillar human type III collagen (Sigma-Aldrich; 100 μg/mL) at a shear rate of 2500 seconds−1 with a syringe pump (Fisher Scientific) for 1 minute. Unbound material was removed by a subsequent perfusion with HEPES-Tyrode buffer for 30 seconds. Thrombus formation was recorded with an inverted epifluorescence microscope (Nikon Eclipse TE2000-U) coupled to the Metamorph Version 7.0rl software (Universal Imaging Corporation) and was quantified by the assessment of the mean percentage of the total area covered by platelets using ImageJ Version 1.44 software (http://rsbweb.nih.gov/ij/index.html). The surface coverage obtained for wt-muVWF (23% ± 4%) was used as a reference and set at 100%.
mAbs to VWF
Three monoclonal antibodies against huVWF were used throughout the study. The first one, mAb 203, blocks binding of VWF to collagen and inhibits platelet adhesion to collagen in flow.13 Its epitope is localized on VWF A3 domain and encompasses residues 1688-1868. mAb 505 is also known to block binding of VWF to collagen24 but is different from mAb 203 because both mAbs do not compete. Its epitope is also localized on the A3 domain and encompasses residues 1690-1877.25 The third mAb tested is mAb 9, which inhibits VWF binding to GPIIbIIIa26 and has an epitope localized between residues 2467 and 2509.25
Binding of anti-VWF antibodies to VWF
Anti-huVWF monoclonal antibodies (mAb 9, mAb 203, and mAb 505) were coated at 5 μg/mL into 96-well Costar plates in carbonate buffer at 4°C overnight. After washing, plasma from VWF−/− mice injected hydrodynamically with the various VWF constructs were incubated in the wells for 2 hours at 37°C. Normal pool plasma from 20 C57BL/6J mice and normal pool plasma from healthy human donors (Cryopep) were used as controls. Detection of binding was done with a horseradish peroxidase–labeled monoclonal anti-VWF antibody directed against the amino-terminal part of the molecule.
Ferric chloride-induced in vivo thrombosis
Ferric chloride injury was induced as described.27 Male and female mice (3-4 weeks old) were anesthetized with an intraperitoneal injection of sodium pentobarbital. Further, animals received rhodamine 6G (3.6 g/kg; Invitrogen) by retro-orbital injection for labeling of platelets in vivo. The mesentery was then exteriorized through a midline abdominal incision, and a single arteriole was selected based on size (90-130 μm) and vessel exposure. A filter paper strip (0.5 × 4 mm) saturated with ferric chloride solution at 7.5% weight/volume (Prolabo) was applied on the surface of the arteriole. After 5 minutes of exposure, the filter paper was removed, and the platelet accumulation was recorded with a 20×/0.4 NA air objective for 40 minutes from the time of filter paper placement. Time to initial thrombus formation with a diameter > 30 μm and time to occlusion were recorded. To study the role of the mAbs directed against human VWF, 100 μg of mAb was administrated by retro-orbital injection 30 minutes before surgical preparation of the mouse. Noninjected mice or mice injected with 100 μg of isotypic antibody were used as a control. Intravital microscopy was performed with a Nikon Eclipse TE2000 microscope (Nikon France) with a 20×/0.4 NA air-objective, and images of thrombus formation were captured using a Sony color video camera 3CCD. Thrombi were analyzed using the Archimed Version 6.1.5 imaging software (Microvision).
Statistics
Wilcoxon-Mann-Whitney rank-sum test, 1-way ANOVA with Dunnett multiple comparison test, and Fisher exact 2-sided test were used as statistical tests in the study. P < .05 was considered significant in all the statistical analyses performed.
Results
Construction of human-murine VWF chimeric molecules
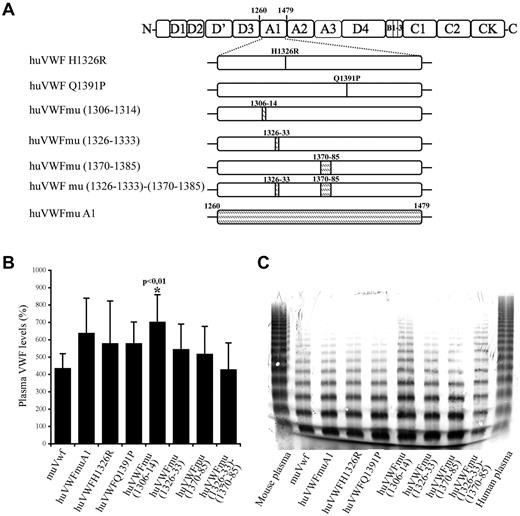
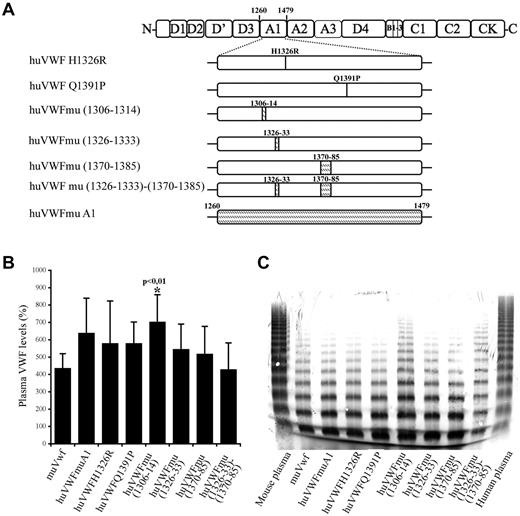
To develop a murine model allowing the in vivo study of drugs targeting human VWF, we first undertook the engineering of a human-mouse chimeric molecule that could bind to murine platelets while retaining most of its human sequence. The choice of the chimeras was based on the crystal structure representing the complex between the A1 domain and GpIb. VWF A1 regions that are in close proximity of the GpIb contact sites include the sequences 1306-1314, 1326-1333, and 1370-1385.28,29 In addition, it has previously been reported that interchanging a single residue at position 1326 (replacing human histidine1326 by murine arginine; huVWF H1326R) would be sufficient to break the interspecies barrier.28 Therefore, we decided to engineer 7 different constructs (Figure 1A). In 2 constructs, a single amino acid was modified: huVWF H1326R and huVWF Q1391P. Six additional constructs were engineered with increasing murine participation until the entire A1 domain of huVWF was replaced by the murine A1 domain (residues 1260-1479).
Construction and characterization of the different human-murine VWF chimeric constructs. (A) Schematic representation of the different VWF constructs with indications of human to murine amino acid changes introduced in human VWF A1 domain. Point mutations and insertions of murine amino acid sequences (shaded area) were done by site-directed mutagenesis. (B) VWF antigen levels after hydrodynamic injection of 150 μg of pCDNA6 containing the different constructs in VWF−/− mice. VWF plasma levels were quantified by ELISA 24 hours after hydrodynamic injection. (C) Multimeric profile of VWF expressed by VWF−/− mice after hydrodynamic injection of the various constructs. Plasma samples were analyzed using SDS/2% agarose gel electrophoresis.
Construction and characterization of the different human-murine VWF chimeric constructs. (A) Schematic representation of the different VWF constructs with indications of human to murine amino acid changes introduced in human VWF A1 domain. Point mutations and insertions of murine amino acid sequences (shaded area) were done by site-directed mutagenesis. (B) VWF antigen levels after hydrodynamic injection of 150 μg of pCDNA6 containing the different constructs in VWF−/− mice. VWF plasma levels were quantified by ELISA 24 hours after hydrodynamic injection. (C) Multimeric profile of VWF expressed by VWF−/− mice after hydrodynamic injection of the various constructs. Plasma samples were analyzed using SDS/2% agarose gel electrophoresis.
In vivo expression of chimeric human/murine VWF variants
Previously, we have shown that expression of wt-muVwf is dependent on the expression plasmid in which it is cloned. To test whether a similar plasmid dependency is also valid for chimeric variants, we determined the time course expression of huVWFmuA1 for both the pCDNA6 and pLIVE plasmid (see supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This analysis revealed that, as for wt-muVwf, expression of huVWFmuA1 peaks after 24 hours when under control of the pCDNA6 CMV promoter and is virtually absent after 96 hours. In contrast, a longer expression is observed when expressed in the context of the pLIVE-plasmid, with > 25% residual expression at 7 days compared with the peak values obtained with this plasmid. For the experiments throughout this study, plasma were used that were taken 1 day after injection with the pCDNA plasmid or plasmas that were taken 4 days after injection with the pLIVE plasmid.
We took advantage of this experiment to also determine endogenous murine ADAMTS13 levels because the hydrodynamic injection procedure may modulate its expression. Indeed, ADAMTS13 levels were reduced to 22% ± 7% at 24 hours after injection, which returned to 48% ± 16% after 96 hours. Thus, the hydrodynamic injection procedure is associated with a transient reduction in ADAMTS13 activity in plasma.
As for the expression levels that were obtained, we observed that all chimeric VWF variants displayed a similar range of expression as wt-muVwf (435% ± 84%) when using the pCDNA6 plasmid (Figure 1B). Only the huVWFmu1306-1314 led to higher levels of expression (702% ± 157%, P < .01). Interestingly, we found that subcloning of the human constructs in pLIVE led to significantly lower levels of expression compared with pCDNA6. For example, injection of huVWFmuA1 cDNA led to expression of 632% ± 210% in pCDNA6 (measured 1 day after injection) and 265% ± 121% in pLIVE (measured 4 days after injection). To avoid the inclusion of mice expressing VWF levels that were too low in the various experiments, VWF antigen was determined for each mouse included in the present study.
To verify the potential effect of the sequence modifications on VWF structure, we analyzed the multimeric profile obtained after hydrodynamic injection of the different constructs in VWF−/− mice (Figure 1C). All constructs led to the presence of multimers, suggesting that the amino acid changes that we introduced were not deleterious. Densitometric analysis revealed the presence of multimer bands between 10 and 15-mers only in 2 constructs, huVWFmu (1306-1314) and huVWFmu (1326-1333; 1370-1385), representing 2% of the total VWF protein loaded on the gel. No differences in multimerization were observed when constructs were subcloned in pCDNA6 or in pLIVE (not shown).
Analysis of the capacity of the different huVWF constructs to support platelet adhesion in flow
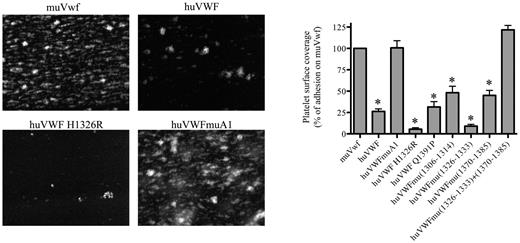
To test the capacity of the human-murine chimeras to bind mouse platelets, we performed perfusion studies in which PPACK-anticoagulated whole blood was perfused over a collagen III surface at 2500 seconds−1. Platelet surface coverage was 23% ± 4% (mean ± SEM) for blood of mice expressing wt-muVwf, and this value was set at 100% as reference (Figure 2). As expected, the presence of human VWF was associated with a significantly reduced surface coverage (25% compared with murine Vwf; P < .0001) because of its inability to efficiently bind mouse platelets. A significant reduction was also observed for most chimeras (5 of 7 tested; Figure 2), some of which displayed even lower surface coverage compared with fully human VWF. A surface coverage similar to wt-muVwf was observed for 2 chimeras: huVWFmuVwf (1326-1333; 1370-1385) that contains 2 short regions of the murine A1 domain and huVWFmuA1 that contains the full murine A1 domain (Figure 2).
Analysis of the capacity of the different human-murine VWF chimeras to promote platelet adhesion in flow. Blood was collected from mice 1 day after hydrodynamic injection with pCDNA6 encoding muVwf, huVWF, or one of the chimeric variants. PPACK-anticoagulated whole blood was incubated with rhodamine 6G to fluorescently label platelets and perfused over collagen-coated glass coverslips (flow rate 2500 seconds−1) for a period of 1 minute. Unbound platelets were removed by subsequent perfusion with HEPES-Tyrode buffer. Thrombus formation was then visualized via image acquisition using Metamorph Version 7.0rl software. Shown are representative images for muVwf, huVWF, huVWF H1326R, and huVWFmuA1. Thrombus formation was quantified using ImageJ Version 1.44 software to calculate percentages of platelet surface coverage. Data represent the mean ± SEM of 3 independent perfusions, with 7-19 images being analyzed for each perfusion. Statistics were performed using 1-way ANOVA followed by the Dunnett multiple comparison test. The various constructs were compared with muVwf, and all except huVWFmu (1326-1333; 1370-1385) and huVWFmuA1 led to *P values < .05.
Analysis of the capacity of the different human-murine VWF chimeras to promote platelet adhesion in flow. Blood was collected from mice 1 day after hydrodynamic injection with pCDNA6 encoding muVwf, huVWF, or one of the chimeric variants. PPACK-anticoagulated whole blood was incubated with rhodamine 6G to fluorescently label platelets and perfused over collagen-coated glass coverslips (flow rate 2500 seconds−1) for a period of 1 minute. Unbound platelets were removed by subsequent perfusion with HEPES-Tyrode buffer. Thrombus formation was then visualized via image acquisition using Metamorph Version 7.0rl software. Shown are representative images for muVwf, huVWF, huVWF H1326R, and huVWFmuA1. Thrombus formation was quantified using ImageJ Version 1.44 software to calculate percentages of platelet surface coverage. Data represent the mean ± SEM of 3 independent perfusions, with 7-19 images being analyzed for each perfusion. Statistics were performed using 1-way ANOVA followed by the Dunnett multiple comparison test. The various constructs were compared with muVwf, and all except huVWFmu (1326-1333; 1370-1385) and huVWFmuA1 led to *P values < .05.
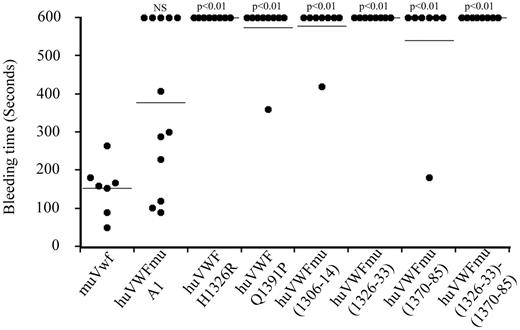
Analysis of the capacity of the different huVWF constructs to correct bleeding time in VWF−/− mice
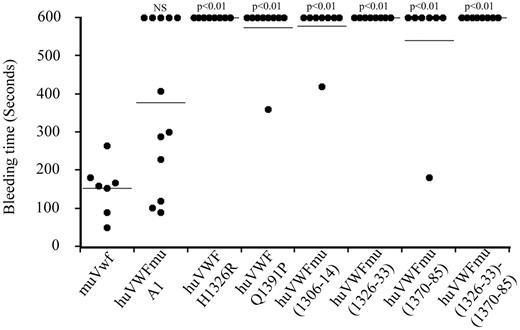
To test whether the lack or ability of the chimeras to support platelet adhesion translated in a corresponding hemostatic response in vivo, we subsequently evaluated the effect of the different modified huVWF molecules in correcting the bleeding time of VWF−/− mice. When VWF−/− mice were injected with muVwf cDNA, all mice were able to control their bleeding in the tail clip assay (152.4 ± 26 seconds vs 600 seconds for nontreated mice; Figure 3). In contrast, mice expressing huVWF H1326R, huVWF Q1391P, huVWFmu (1306-14), huVWFmu (1326-33), huVWFmu (1370-85), or huVWFmu (1326-33; 1370-85) did not show any significant improvement of their bleeding phenotype. For mice expressing the huVWFmuA1, 7 of 12 mice were able to stop bleeding, a result that was not statistically different from muVwf (Figure 3). By correlating expression levels and capacity of the construct to correct bleeding time, we were able to establish that a threshold of 300% VWF:Ag is necessary for muVwf whereas a plasma level of 500% is required for huVWFmuA1 to be efficient. For all other human-murine VWF molecules, even high expression levels proved inefficient in restoring hemostasis.
Analysis of the capacity of the different human-murine VWF chimeras to correct bleeding time of VWF−/− mice. Eight-week old VWF−/− mice received 150 μg of pCDNA6 containing the different constructs by hydrodynamic injection. Bleeding tests were done 24 hours after injection by the tail clip assay. Observation was stopped at 600 seconds when bleeding did not cease. Each dot represents an individual mouse, and the horizontal line represents the mean bleeding time per group. Statistical analysis was done using Fisher exact 2-sided test by comparison with muVwf. NS indicates not significant.
Analysis of the capacity of the different human-murine VWF chimeras to correct bleeding time of VWF−/− mice. Eight-week old VWF−/− mice received 150 μg of pCDNA6 containing the different constructs by hydrodynamic injection. Bleeding tests were done 24 hours after injection by the tail clip assay. Observation was stopped at 600 seconds when bleeding did not cease. Each dot represents an individual mouse, and the horizontal line represents the mean bleeding time per group. Statistical analysis was done using Fisher exact 2-sided test by comparison with muVwf. NS indicates not significant.
Effect of huVWFmuA1 chimera in thrombus formation
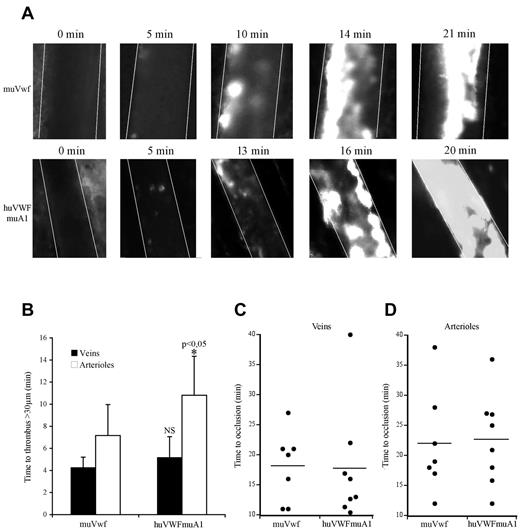
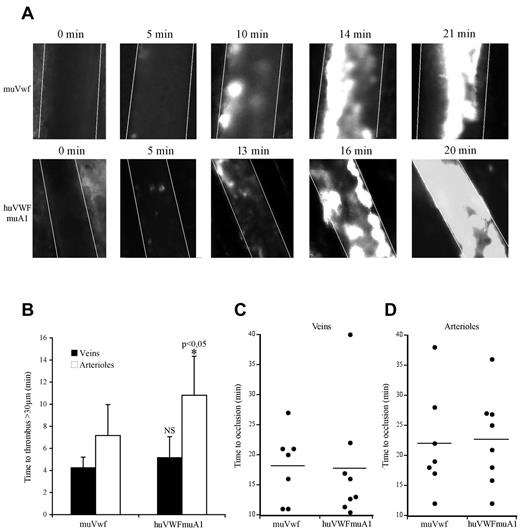
Because only the huVWFmuA1 chimera appeared efficient in correcting bleeding time in VWF−/− mice, we next focused on this particular molecule to evaluate its potential in a murine thrombosis model. We used the pLIVE-encoded huVWFmuA1 construct because the longer expression kinetic allowed us to perform our experiments 3-4 days after hydrodynamic injection. We compared thrombus formation in VWF−/− mice expressing either huVWFmuA1 or muVwf. In both sets of mice, platelets adhered to the injured vessel wall and ultimately formed an occlusive thrombus (Figure 4A). The time necessary to form a first thrombus > 30 μm was 4.2 ± 0.9 minutes for veins and 7.1 ± 2.8 minutes for arterioles in mice expressing muVwf. In mice expressing huVWFmuA1, time to form a > 30-μm thrombus was similar in veins (5.18 ± 1.9 minutes) but slightly prolonged in arterioles (10.8 ± 3.5 minutes; P = .048; Figure 4B). However, occlusion times were not different between muVwf or huVWFmuA1-expressing mice (18.3 ± 5.9 minutes vs 17.8 ± 9.7 minutes in veins, and 22.1 ± 8.6 minutes vs 22.7 ± 7.6 minutes in arterioles, respectively; Figure 4C-D). These results confirm the functionality of the huVWFmuA1 in the mouse after hydrodynamic injection, thus validating this model for studying the effect of anti–human VWF molecules as antithrombotics in vivo.
Comparative effect of huVWFmuA1 versus muVwf in thrombus formation in vivo. Four-week-old VWF−/− mice received 150 μg of pLIVE plasmid containing either huVWFmuA1 (n = 8) or control muVwf (n = 7) cDNA by hydrodynamic injection. Four days after, in vivo thrombosis was assessed after exposure of mesenteric vessels to 7.5% FeCl3 for 5 minutes. Platelets were labeled in vivo through injection of rhodamine 6G before mesentery dissection. Platelet accumulation was recorded during 40 minutes or until final occlusion of the vessel. (A) Photographs of arterioles from muVwf and huVWFmuA1-expressing mice at different time points after FeCl3 exposure. (B) Time necessary to form a thrombus > 30 μm in veins or arterioles of mice expressing muVwf or huVWFmuA1. *P < .05 in arterioles of huVWFmuA1-expressing mice compared with muVwf-expressing mice. Statistical analysis was done using Wilcoxon-Mann-Whitney rank-sum test. (C-D) Occlusion time in veins (C) and arterioles (D) of muVwf and huVWFmuA1-expressing mice. Result from an individual mouse is represented by a dot, and a horizontal line represents the mean time of occlusion for each group. Statistical analysis was done with Fisher exact 2-sided test.
Comparative effect of huVWFmuA1 versus muVwf in thrombus formation in vivo. Four-week-old VWF−/− mice received 150 μg of pLIVE plasmid containing either huVWFmuA1 (n = 8) or control muVwf (n = 7) cDNA by hydrodynamic injection. Four days after, in vivo thrombosis was assessed after exposure of mesenteric vessels to 7.5% FeCl3 for 5 minutes. Platelets were labeled in vivo through injection of rhodamine 6G before mesentery dissection. Platelet accumulation was recorded during 40 minutes or until final occlusion of the vessel. (A) Photographs of arterioles from muVwf and huVWFmuA1-expressing mice at different time points after FeCl3 exposure. (B) Time necessary to form a thrombus > 30 μm in veins or arterioles of mice expressing muVwf or huVWFmuA1. *P < .05 in arterioles of huVWFmuA1-expressing mice compared with muVwf-expressing mice. Statistical analysis was done using Wilcoxon-Mann-Whitney rank-sum test. (C-D) Occlusion time in veins (C) and arterioles (D) of muVwf and huVWFmuA1-expressing mice. Result from an individual mouse is represented by a dot, and a horizontal line represents the mean time of occlusion for each group. Statistical analysis was done with Fisher exact 2-sided test.
Interaction of the huVWFmuA1 chimera with anti–human VWF monoclonal antibodies
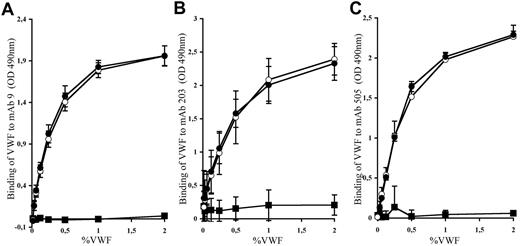
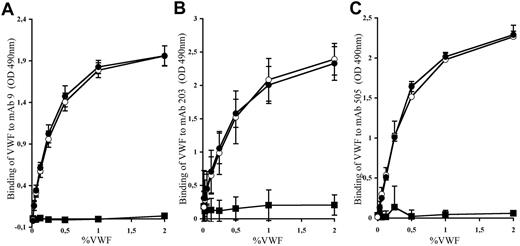
Before testing the effect of mAbs against human VWF in thrombosis, we first needed to validate that such antibodies were able to recognize the human-mouse VWF chimera. We thus measured the binding of the different mAbs (mAb 9, mAb 203, and mAb 505) to huVWF, muVwf, and huVWFmuA1 expressed in plasma of VWF−/− mice. As expected, binding of the different mAbs to huVWF showed a dose-response curve (Figure 5). No unspecific binding toward muVwf was obtained, confirming the specificity of the different mAbs toward huVWF molecule. Interaction of mAb 9, mAb 203, and mAb 505 to huVWFmuA1 was dose-dependent and very similar to that obtained for huVWF. Our results show that replacement of the human A1 domain with its murine counterpart does not modify the recognition of VWF by the selected mAbs.
Binding of mAbs to huVWF, muVwf, and huVWFmuA1. Plasma from VWF−/− mice expressing huVWFmuA1 (○), huVWF (●), or muVwf (■) was evaluated for binding with (A) mAb9, which blocks the interaction of human VWF with GPIIbIIIa; (B) mAb203, which blocks the interaction of human VWF with fibrillar collagens; and (C) mAb505, which also interferes with the interaction of human VWF with fibrillar collagen but is different from mAb203. The tested antibodies were immobilized on a microtiter plate, and plasma was added at different VWF concentrations. Bound VWF was revealed with an HRP-labeled anti-VWF antibody. Each graph represents the mean ± SEM of the absorbance at 490 nm from 3 independent experiments.
Binding of mAbs to huVWF, muVwf, and huVWFmuA1. Plasma from VWF−/− mice expressing huVWFmuA1 (○), huVWF (●), or muVwf (■) was evaluated for binding with (A) mAb9, which blocks the interaction of human VWF with GPIIbIIIa; (B) mAb203, which blocks the interaction of human VWF with fibrillar collagens; and (C) mAb505, which also interferes with the interaction of human VWF with fibrillar collagen but is different from mAb203. The tested antibodies were immobilized on a microtiter plate, and plasma was added at different VWF concentrations. Bound VWF was revealed with an HRP-labeled anti-VWF antibody. Each graph represents the mean ± SEM of the absorbance at 490 nm from 3 independent experiments.
Role of antibodies against VWF in thrombus formation in vivo
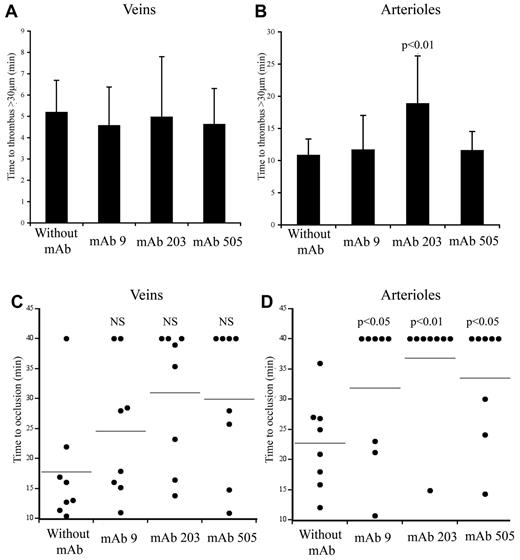
We next evaluated how mAbs blocking VWF binding to collagens or GPIIbIIIa would interfere with thrombus formation in vivo. VWF−/− mice expressing the chimeric molecule huVWFmuA1 were injected with the different mAbs 30 minutes before thrombosis induction with FeCl3.
Inhibition of VWF-collagen interaction in thrombus formation
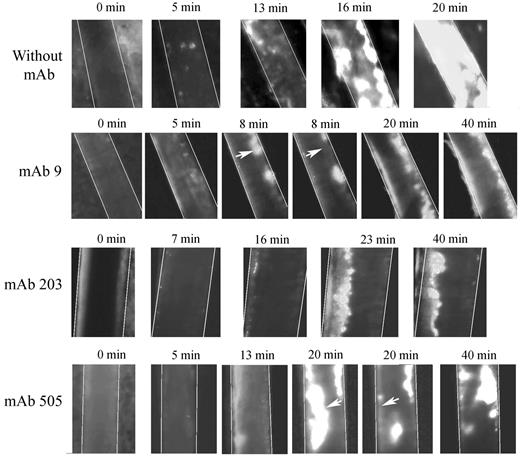
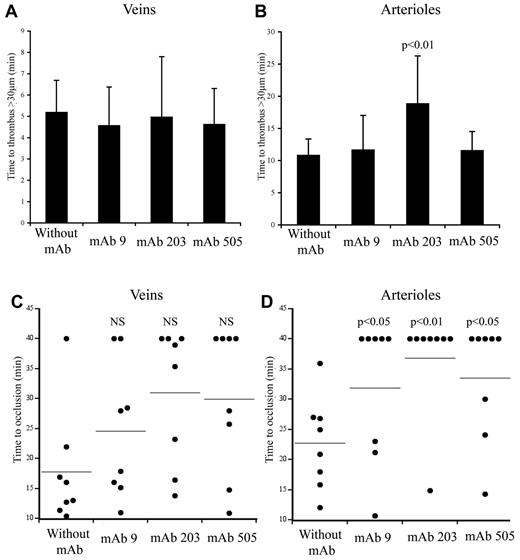
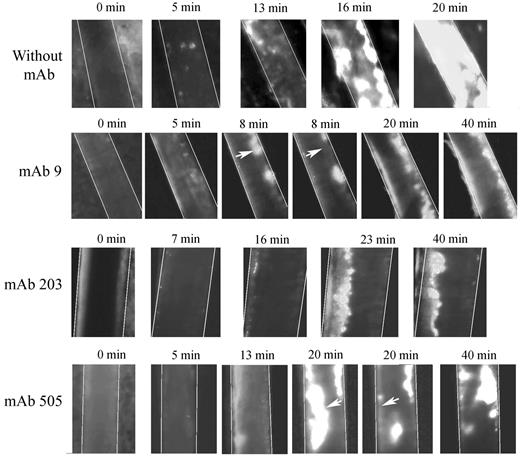
We used 2 different mAbs blocking VWF-collagen interaction (mAbs 203 and 505) developed in our laboratory. Although they both recognize the VWF A3 domain, they do not compete with each other for binding to VWF. In veins, initial thrombus formation was not affected by the presence of the mAbs. Time necessary to form a > 30-μm thrombus was similar in untreated mice (5.2 ± 1.87 minutes) compared with mice pretreated with mAb 203 (5.0 ± 3.4 minutes) or with mAb 505 (4.6 ± 2.1 minute; Figure 6A). Similarly, time to occlusion in veins was not significantly affected in the presence of these mAbs, despite a trend toward more mice not reaching occlusion (Figure 6C). In arterioles, only mice treated with mAb 203 showed a delayed time to form a thrombus > 30 μm (18.8 ± 9.2 minutes vs 10.8 ± 3.5 minutes in control mice, P = .014; Figure 6B). Occlusion in arterioles was severely affected in mice treated either with mAb 203 (only 1 of 8 mice reaching occlusion) or mAb 505 (3 of 8 mice reaching occlusion) compared with control mice (8 of 8 mice reaching occlusion; Figure 6D). Qualitatively, both antibodies had different effects. Initial platelet adhesion was delayed in mice treated with mAb 203 (Figure 7). Moreover, thrombus growth was very slow and occlusion was not reached during the 40-minute observation period (Figure 7). However, this thrombus was rather stable and no embolization was observed. In contrast, in mice treated with mAb 505, initial platelet adhesion was not significantly delayed but embolization was frequently observed (arrows, Figure 7), preventing full occlusion of the vessel. Treatment of mice with control IgG led to results similar to untreated mice.
Effect of mAbs to VWF in thrombus formation in mice expressing huVWFmuA1. Four- to 5-week-old VWF−/− mice were hydrodynamically injected with 150 μg of pLIVE huVWFmuA1 cDNA by hydrodynamic injection. Four days later, mice were injected with 100 μg/mouse of mAb 9 (recognizing RGD-motif), mAb 203 or mAb 505 (both recognizing A3 domain), and thrombosis was induced by 7.5% FeCl3 exposure 30 minutes after mAb injection. The time necessary to form a thrombus of > 30 μm in veins (A) and in arterioles (B) was recorded. Statistical analysis was done using Wilcoxon-Mann-Whitney rank-sum test. Time to occlusion in veins (C) and arterioles (D) was also measured. NS indicates not significant. Results from individual mice are represented by a dot, and a horizontal line represents the average occlusion time for each group. Statistical analysis was done with Fisher exact 2-sided test.
Effect of mAbs to VWF in thrombus formation in mice expressing huVWFmuA1. Four- to 5-week-old VWF−/− mice were hydrodynamically injected with 150 μg of pLIVE huVWFmuA1 cDNA by hydrodynamic injection. Four days later, mice were injected with 100 μg/mouse of mAb 9 (recognizing RGD-motif), mAb 203 or mAb 505 (both recognizing A3 domain), and thrombosis was induced by 7.5% FeCl3 exposure 30 minutes after mAb injection. The time necessary to form a thrombus of > 30 μm in veins (A) and in arterioles (B) was recorded. Statistical analysis was done using Wilcoxon-Mann-Whitney rank-sum test. Time to occlusion in veins (C) and arterioles (D) was also measured. NS indicates not significant. Results from individual mice are represented by a dot, and a horizontal line represents the average occlusion time for each group. Statistical analysis was done with Fisher exact 2-sided test.
Representative images of thrombus formation in arterioles from huVWFmuA1-expressing mice treated with mAbs to VWF. Four- to 5-week-old VWF−/− mice expressing huVWFmuA1 were treated with 100 μg/mouse of mAb 9 (recognizing RGD-motif), mAb 203 or mAb 505 (both recognizing A3 domain) 4 days after hydrodynamic injection. Platelets were labeled in vivo through injection of rhodamine 6G before mesentery dissection. Vessel injury was induced by exposure of arterioles to 7.5% FeCl3. The experiment was recorded until occlusion or until 40 minutes in absence of occlusion. Pictures were taken at different time points after induction of vessel wall injury. White arrows point to embolization.
Representative images of thrombus formation in arterioles from huVWFmuA1-expressing mice treated with mAbs to VWF. Four- to 5-week-old VWF−/− mice expressing huVWFmuA1 were treated with 100 μg/mouse of mAb 9 (recognizing RGD-motif), mAb 203 or mAb 505 (both recognizing A3 domain) 4 days after hydrodynamic injection. Platelets were labeled in vivo through injection of rhodamine 6G before mesentery dissection. Vessel injury was induced by exposure of arterioles to 7.5% FeCl3. The experiment was recorded until occlusion or until 40 minutes in absence of occlusion. Pictures were taken at different time points after induction of vessel wall injury. White arrows point to embolization.
Inhibition of VWF-GPIIbIIIa interaction in thrombus formation
mAb 9, developed in our laboratory, is a potent inhibitor of VWF-GPIIbIIIa interaction.26 In mice expressing huVWFmuA1 and treated with this mAb, no delay in time to form a > 30-μm thrombus could be observed in veins (4.6 ± 2.2 minutes) or in arterioles (11.6 ± 6.4 minutes) compared with control mice (Figure 6A-B). In terms of occlusion, there was no significant difference in veins (6 of 8 mice reaching occlusion vs 7 of 8 for control mice; Figure 6C). However, in arterioles, occlusion was significantly impaired (with only 3 of 8 mice reaching occlusion; Figure 6D). Recurrent embolization was observed in mice treated with mAb 9 (arrows, Figure 7). Actually, in the 3 mice where arteriolar occlusion was observed, it was the embolization phenomenon that led to occlusion downstream of the injured portion of the vessel.
Discussion
Targeting VWF is an increasingly recognized strategy to fight arterial thrombosis, mostly because of its specific high shear stress dependent function in platelet adhesion. Presently, 2 different drugs, an aptamer and a nanobody, are being investigated in phase 2 clinical trials. Both these drugs target the VWF-GPIb interaction, therefore inhibiting the very first step of platelet recruitment in arteriolar conditions. However, the risk of bleeding events associated with such an approach should not be underestimated, even if preliminary studies showed this risk to be decreased compared with drugs targeting the platelet GPIIbIIIa receptor.7 Using a VWF mutant unable to bind to GPIb, we have shown that mice expressing such a mutant were indeed protected against thrombosis, but they also showed a hemorrhagic phenotype.15 In that same study, we also studied mice expressing VWF mutants unable to bind to fibrillar collagens or to GPIIbIIIa and noticed that these mice had decreased thrombosis while retaining full hemostatic capacity.15 Based on these results, we decided to investigate the antithrombotic potential of specific tools targeting these interactions. A humanized antibody inhibiting VWF binding to collagens, mAb 82D6A3, has been studied in the past and was shown to inhibit thrombus formation in baboons using the Folt model10 but proved inefficient in preventing restenosis.30 Targeting the VWF-GPIIbIIIa interaction has never been investigated in in vivo models. This last point is representative of the difficulty to test inhibitors of VWF function in vivo. Indeed, all the tools that have been developed to inhibit VWF are specific of huVWF, and there are no easily accessible animal models in which huVWF is still functional. In mice, which now represent a choice model to study thrombosis, huVWF is particularly inefficient in binding to murine platelet GpIb and is therefore nonfunctional in vivo. However, the possibility to express genetically modified VWF variants via hydrodynamic gene transfer offers an opportunity to create a model in which functional chimeric human/murine VWF variants are being expressed. Despite the intrinsic limitations of this approach (reduced ADAMTS13 levels, which may promote thrombus formation; absence of ultra-large VWF molecules, which may render thrombus formation less efficient; constitutive expression in hepatocytes rather than regulated secretion from endothelial cells and platelets), the hydrodynamic gene transfer approach has been shown to be a powerful tool for the in vivo analysis of VWF genotype-phenotype relationships.15,16,27
One of our main objectives was to bypass the human/murine species barrier by developing a model where huVWF would be modified to be able to bind to murine platelets. The opposite experiment, making muVwf able to bind to human platelets, was possible thanks to a single amino acid replacement in muVwf, Arg 1326 to His.28 This group further reported that the opposite approach (replacing His1326 by Arg in huVWF) resulted in a human A1 domain protein that promoted binding of mouse platelets.28 Unfortunately, introducing this replacement in the full-length huVWF molecule proved insufficient to convert huVWF in a protein that interacts with mouse platelets, either in vitro or in vivo (Figures 2 and 3). Therefore, a series of additional chimeric variants were constructed in which different parts of the murine A1 domain were incorporated. Besides those that were tested in this study (depicted in Figure 1A), we also tested 4 additional constructs, but their low expression levels obtained on injection in mice precluded further analysis.
In in vitro perfusion assays of whole blood over collagen-coated surface, wt-huVWF proved 4-fold less efficient than wt-muVwf in supporting platelet adhesion. These data confirm that huVWF is able to bind murine platelets, but rather inefficiently. Indeed, very high concentrations of human VWF that contains ultra-large VWF multimers are needed to induce thrombocytopenia thrombotic purpura-like symptoms in ADAMTS13-deficient mice.31 Apart from wt-huVWF, also other chimeras were inefficient in promoting thrombus formation, with the exception of 2, huVWFmu (1326-1333; 1370-1385) and huVWFmuA1, both of which were similar to wt-muVwf in promoting thrombus formation in this in vitro flow system.
Interestingly, huVWFmu (1326-1333; 1370-1385) was unable to support hemostasis in the tail clip assay, despite its efficiency in vitro. The reason why this in vitro efficacy is not translated in an in vivo hemostatic activity remains unclear. In contrast, the huVWFmuA1 was able to support hemostasis in vivo, not only in the tail clip assay but also in the ferric chloride-induced vascular injury model (Figures 3 and 4). Of note, higher expression levels were needed for mice to stop bleeding. Because both wt-muVwf and the huVWFmuA1 chimera appeared to be multimerized at a similar level (Figure 1C), such a difference may potentially be explained by a decreased affinity of huVWFmuA1 for the murine collagens or GPIIbIIIa.
However, most relevant to our study was the observation that huVWFmuA1 proved functional in the FeCl3-induced thrombosis model, thus validating this mouse model as a tool allowing the evaluation of different antithrombotic molecules targeting huVWF.
The presence of the entire muA1 domain in our chimera prevented us from testing mAbs inhibiting the VWF-GPIb interaction because the epitopes of these mAbs are located in the A1 domain and none of the mAbs available to us cross-reacted with muA1. However, our model was perfectly suitable to evaluate antithrombotic activity of mAbs inhibiting VWF interaction with collagen (mAbs 203 and 505) or GPIIbIIIa (mAb 9). Concerning these mAbs, no problem of recognition toward our chimeric molecule was observed, as their binding to huVWFmuA1 was similar to the binding observed for huVWF. When injected before vessel injury by FeCl3, all 3 antibodies were able to significantly delay vessel occlusion in arterioles of VWF−/− mice expressing the huVWFmuA1 chimera. No effect was visible in veins, suggesting that, in these conditions, VWF interaction with collagens or with GPIIbIIIa is not critical for thrombus formation and that platelets can use alternative pathways to adhere and aggregate. In contrast, in conditions found in arterioles, blocking these interactions is clearly deleterious for the thrombotic process. Not all antibodies were similar in their mode of action. mAb 203 was the most potent and strongly delayed thrombus growth, whereas mAb 505 and mAb 9 seemed to interfere with thrombus stability. The fact that 2 mAbs (203 and 505) inhibiting the same interaction display distinct antithrombotic effect is particularly interesting and may find its source in the different epitopes for these mAbs. Interestingly, interaction of VWF with bitiscetin, a snake venom protein inducing a conformational change of the A3 domain leading to VWF interaction with GPIb in vitro, is inhibited by mAb 203 and not by mAb 505, emphasizing the dissimilarity between both mAbs.32
The potential antithrombotic effect of an antibody blocking VWF-GPIIbIIIa interaction had never been tested in vivo before and such a tool held the potential to be safer than anti-GPIIbIIIa agents in terms of hemorrhagic risk because it would display high shear rate selectivity. Indeed, the thrombotic process was decreased in mice treated with mAb 9 or in mice expressing a mutant VWF unable to bind to GPIIbIIIa.15 However, at least in the mouse model, inhibiting VWF-GPIIbIIIa interaction induces frequent embolization, which may lead to downstream vessel occlusion, a highly undesirable phenomenon.
In conclusion, we have developed a mouse model, in which by transiently expressing a human-murine VWF chimera, it is possible to test the efficacy of various agents targeting huVWF. As a proof of concept, we have evaluated 3 different anti-huVWF mAbs blocking VWF interaction with collagens or with GPIIbIIIa. Our results confirm the antithrombotic potential of blocking VWF interaction with collagens.33,34 Using our easily accessible model, it will be possible to test optimized tools targeting this early step in platelet adhesion and thrombus formation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by Inserm and Agence Nationale de la Recherche (ANR-08-EBIO-026-01, C.V.D.; and ANR-08-CEXC-018-01, P.J.L.). C.C. received a postdoctoral grant from the Fondation de la Recherche Médicale.
Authorship
Contribution: A.-M.N., C.C., P.L., I.M., J.-R.H., O.D.C., and C.V.D. performed experiments and analyzed data; P.J.L., O.D.C., and C.V.D. designed research; A.-M.N., P.J.L., and C.V.D. wrote manuscript; and all authors contributed to the editing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cécile V. Denis, Inserm U770, 80 rue du General Leclerc, 94276 Le Kremlin-Bicêtre, France; e-mail: cecile.denis@inserm.fr.