The 1989 identification of HCV as the cause of non-A, non-B hepatitis led to serologic and nucleic acid tests for HCV that have dramatically reduced the transmission of HCV through blood and blood products. Still, at least 130 million people worldwide have persistent HCV infection,2 which can cause chronic liver disease, cirrhosis, hepatocellular carcinoma, and extrahepatic diseases.3 Infection continues to spread through unsafe injection practices; vertical, nosocomial, and sexual transmission also occur. HCV has surpassed HIV as a cause of death in the United States4 and is the leading indication for liver transplant in the Western world. Major advances have been made in antiviral treatment,5 but no vaccine is yet available for HCV infection.6 The development of a vaccine is a daunting challenge, given HCV's impressive genetic diversity.
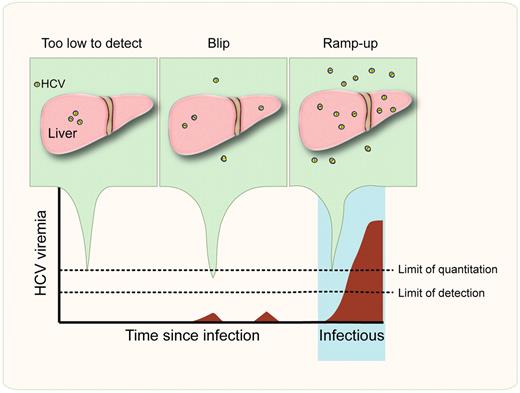
The earliest stages of hepatitis C virus (HCV) infection. During the eclipse phase, HCV replicates in hepatic foci but may be only sporadically detectable as “blips” of viremia in the blood; levels of HCV RNA are too low to quantify using licensed clinical assays. HCV is infectious just before or at the start of the ramp-up phase, when circulating viral loads begin to increase exponentially.
The earliest stages of hepatitis C virus (HCV) infection. During the eclipse phase, HCV replicates in hepatic foci but may be only sporadically detectable as “blips” of viremia in the blood; levels of HCV RNA are too low to quantify using licensed clinical assays. HCV is infectious just before or at the start of the ramp-up phase, when circulating viral loads begin to increase exponentially.
Acute HCV infection is often asymptomatic, and seroconversion may take 2 or more months after the onset of high-level viremia (reviewed in Dustin and Rice7 ). Identification of acute HCV infection has important clinical implications, both for prevention of transmission and to take advantage of a favorable window for antiviral therapy.8 The interval between contamination and development of high-level viremia has been characterized in panels of repeat plasma donors,9 revealing similarities to patterns described for other pathogens such as HIV (see figure). There is an eclipse period lasting days to several weeks, during which HCV RNA is not detectable in the blood although the virus has likely established a foothold in susceptible cells. Testing of multiple replicates during the eclipse phase may reveal occasional “blips” of HCV RNA representing very low levels of viremia. Blips are detected by qualitative HCV assays but RNA levels are too low to be quantified. The eclipse phase is followed by an exponential increase in HCV RNA, termed the ramp-up. During the ramp-up period, viral RNA levels become readily detectable by qualitative and quantitative nucleic acid testing.
The study by Busch and colleagues addresses an important question: how well does nucleic acid testing for HCV prevent transmission of infectious virus? In this study, plasma lots obtained from repeat donors who subsequently developed viremia were tested for the ability to transmit HCV infection. In clever experiments designed to maximize the information obtained from 2 chimpanzees, the authors administered 50-mL aliquots of plasma from multiple eclipse-phase donors.1 Because the donors later became viremic with disparate HCV strains, this approach allowed the investigators to trace the source of any infection in the recipient animal. A plasma lot obtained just before the ramp-up period was infectious. The investigators calculated, after testing the infectious lot more than 2 dozen times, that the transfused plasma contained approximately 60 copies of viral RNA. Plasma samples collected from the same donor during valley periods when no viral RNA was detectable, and during blips of viremia, were not infectious in a second animal. Why was plasma containing similar numbers of viral RNA copies infectious in the ramp-up period, but not earlier? Perhaps during early infection, the ratio of susceptible cells to the number of functional HCV virions is so high that HCV readily spreads from cell to cell without much spillover into the blood; thus, the small amount of HCV RNA observed in the blood at this stage may represent defective, noninfectious particles.
Although the study is limited in size and scope, it also highlights the challenges of HCV vaccine development. Like 20% to 50% of humans who contract HCV infection,10 both chimpanzees spontaneously recovered and were free of detectable virus. The immunologic mechanisms behind spontaneous viral clearance are not fully understood. Those who recover from HCV infection, whether or not they undergo antiviral treatment, are not reliably protected against re-infection by similar or disparate HCV strains.7 New strategies to test HCV vaccines are urgently needed as ethical restrictions limit the use of chimpanzees in medical research.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health