Abstract
We report the results of a prospective, patient self-selected study evaluating whether haploidentical related donor stem cell transplantation (HRD-HSCT) is superior to chemotherapy alone as postremission treatment for patients with intermediate- or high-risk acute myeloid leukemia (AML) in first complete remission (CR1). Among totally 419 newly diagnosed AML patients, 132 patients with intermediate- and high-risk cytogenetics achieved CR1 and received chemotherapy alone (n = 74) or HSCT (n = 58) as postremission treatment. The cumulative incidence of relapse at 4 years was 37.5% ± 4.5%. Overall survival (OS) and disease-free survival (DFS) at 4 years were 64.5% ± 5.1% and 55.6% ± 5.0%, respectively. The cumulative incident of relapse for the HRD-HSCT group was significantly lower than that for the chemotherapy-alone group (12.0% ± 4.6% vs 57.8% ± 6.2%, respectively; P < .0001). HRD-HSCT resulted in superior survival compared with chemotherapy alone (4-year DFS, 73.1% ± 7.1% vs 44.2% ± 6.2%, respectively; P < .0001; 4-year OS, 77.5% ± 7.1% vs 54.7% ± 6.3%, respectively; P = .001). Multivariate analysis revealed postremission treatment (HRD-HSCT vs chemotherapy) and high WBC counts at diagnosis as independent risk factors affecting relapse, DFS, and OS. Our results suggest that HRD-HSCT is superior to chemotherapy alone as postremission treatment for AML.
Introduction
Acute myeloid leukemia (AML) is a common malignant clonal disease of hematopoietic stem cells. Allogeneic HLA-identical hematopoietic stem-cell transplantation (allo-HSCT) is one of the best options to cure AML and offers significantly improved overall survival (OS) for intermediate- and high-risk patients.1-9 Recent advances in HLA-mismatched/haploidentical transplantation allow for the use of HSCT in many patients without an HLA-identical donor or who urgently require transplantation.10-17 Rizzieri et al exploited a nonmyeloablative conditioning regimen incorporating fludarabine and cyclophosphamide (CY) plus alemtuzumab in 49 hematologic malignancy patients receiving peripheral blood stem cells from HLA-haploidentical donors, resulting in 31% OS for patients 1 year after transplantation.14 Ogawa et al reported a study of 30 patients with advanced-stage hematologic malignancies or with poor prognosis who received unmanipulated HLA 2-3 antigen-mismatched transplantation, and in that study the 3-year survival rate was 49.9%.15 Available data suggest that haploidentical HSCT is a feasible treatment option for high-risk AML patients who lack an HLA-matched donor.10,17-19
Researchers at Peking University developed the GIAC protocol, a novel approach to HLA-mismatched/haploidentical related donor HSCT (HRD-HSCT) without in vitro T-cell depletion.11-13 (The GIAC protocol entails the following: treating donors with granulocyte colony-stimulating factor [G-CSF] to induce donor immune tolerance, intensified immunologic suppression to both promote engraftment and to prevent GVHD, antithymocyte globulin [ATG] was included for the prophylaxia of GVHD and graft rejection, and combination of G-CSF–primed bone marrow harvest [G-BM] and G-CSF–mobilized peripheral blood stem cell harvest [G-PB] as the source of stem cell grafts.) Compared with HLA-matched sibling donor (MSD) transplantation, similar relapse rates, transplant-related mortality (TRM), leukemia-free survival (LFS), and OS were achieved using the GIAC protocol.12 Promising results were also seen after treating AML with haploidentical HSCT without T-cell depletion; the 3-year probabilities of LFS were 70.7% and 55.9% in the standard-risk and high-risk groups, respectively. In a historic comparison of 117 consecutive, high-risk acute leukemia patients undergoing HSCT from HLA-mismatched/haploidentical donors (HIDs; n = 81) or HLA-identical sibling donors (ISDs; n = 36) without in vitro T-cell depletion, Wang et al found that the 3-year OS rate was higher in HID patients (42%) than in ISD (20%) patients (P = .048).19 The investigators suggested that HID transplantations can achieve a stronger GVL effect than ISD transplantation for high-risk acute leukemia patients.
Although the effects of HLA-matched allo-HSCT on patients with AML in first complete remission (CR1) are well established,1,2,4,6-8,20 the role of haploidentical HSCT in AML is not well defined. In the present study, we investigated whether haploidentical HSCT as postremission treatment in patients with intermediate- and high-risk AML has a favorable impact on survival by comparing the effects with those of chemotherapy alone in a prospective clinical study.
Methods
Patients
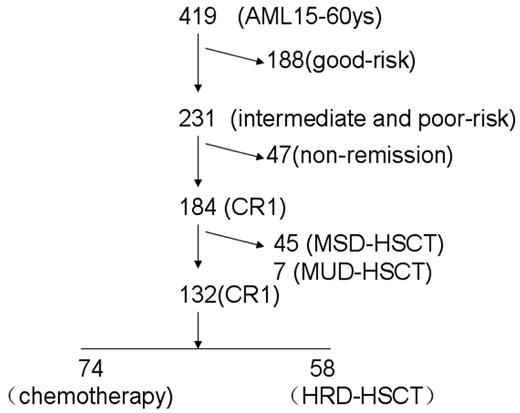
From January 2006 until May 2010, newly diagnosed AML patients were enrolled in this study. To meet the inclusion criteria, patients had to meet the following characteristics: (1) be between the ages of 15 and 60 years, (2) have intermediate- or high-risk AML based on cytogenetics, (3) be in CR1, and (4) have no contraindications to consolidation chemotherapy or HSCT. Patients who received MSD-HSCT or matched unrelated donor HSCT (MUD-HSCT) were excluded from the analysis. For each patient, informed consent was obtained from patients or guardians in accordance with the Declaration of Helsinki. The trial was approved by the ethical committee at the Peking University People's Hospital. Figure 1 provides an overview of the enrolled patients, including risk classification, HLA typing, and donor availability.
Overview of patients included in the analysis by risk classification, HLA typing, and donor availability. AML indicates acute myeloid leukemia; CR, complete remission; HSCT, hematopoietic stem-cell transplantation. HRD, haploidentical related donor; MSD, matched sibling donor; and MUD, matched unrelated donor.
Overview of patients included in the analysis by risk classification, HLA typing, and donor availability. AML indicates acute myeloid leukemia; CR, complete remission; HSCT, hematopoietic stem-cell transplantation. HRD, haploidentical related donor; MSD, matched sibling donor; and MUD, matched unrelated donor.
Diagnostics and assessment of treatment response
AML diagnosis was performed as described previously.1,21 Cytogenetic studies were carried out using standard techniques. Molecular screening for fusion genes was offered to all patients. CR was defined as BM blasts < 5%, absence of blasts with Auer rods, absence of extramedullary disease, absolute neutrophil count > 1.0 × 109/L, platelet (PLT) count > 100 × 109/L, and absence of RBC transfusions. Relapse was defined as recurrence of BM blasts ≥ 5%, reappearance of blasts in the blood, or development of extramedullary disease infiltrates at any site.
Definitions of risk groups
Patients were classified as standard-, intermediate-, or high-risk on the basis of cytogenetic abnormalities. Cytogenetic abnormalities t(8;21)(q22;q22), t(15;17)(q22;q21), and inv(16) or t(16;16)(p13;q22) were considered standard-risk. Complex cytogenetic abnormalities (defined as at least 3 unrelated cytogenetic clones) and −5/5q−, −7/7q−, abn(3q), t(6;9)(q23;q34), abn(11q23), and t(9;22)(q34;q11) were considered high-risk. Patients without favorable or adverse abnormalities or without karyotype information were classified as intermediate-risk.22
Treatment protocols
Treatment involved 1-2 cycles of induction with an anthracycline (daunorubicin 45 mg/m2 or idarubicin 8-10 mg/m2 for 3 days) in combination with cytarabine (100 mg/m2 for 7 days). If CR occurred, patients received consolidation therapy. Patients in the chemotherapy alone group received 2 cycles of intermediate-dose cytarabine (1-2 g/m2 every 12 hours for 3 days) and 6-8 cycles of anthracycline (daunorubicin 45 mg/m2 or idarubicin 8-10 mg/m2 for 3 days, homoharringtonine 2 mg/m2 for 7 days, mitoxantrone 8 mg/m2 for 3 days, or aclamycin 20 mg/d for 7 days) plus cytarabine (100 mg/m2 for 7 days) chemotherapy. When a suitable donor was available, eligible patients proceeded to HSCT after receiving 2-4 cycles of consolidation therapy including 1-2 cycles of intermediate-dose cytarabine and 1-2 cycles of anthracycline plus cytarabine-based chemotherapy.
For HRD-HSCT patients, as described previously,11,12,18 G-CSF–mobilized BM and peripheral blood stem cells were used as a graft resource. The conditioning regimen was a modification of BU/CY (busulfan/cyclophosphamide) and antithymocyte globulin (ATG), which consisted of the following: cytosine arabinoside (4 g/m2/d IV) on days −10 and −9; busulfan (12 mg/kg orally in 12 doses) on days −8, −7, and −6; cyclophosphamide (1.8 g/m2/d IV) on days −5 and−4; semustine (250 mg/m2, orally) on day −3, and thymoglobulin (rabbit ATG [Sangstat-Genzyme] 2.5 mg/kg/d IV or porcine ATG [Bioproduct Inc] 20 mg/kg/d) through days −5 to −2. All transplantation recipients received cyclosporine A (CsA), mycophenolate mofetil (MMF), and short-term methotrexate (MTX) as GVHD prophylaxis. The dosage of CsA was 2.5 mg/kg/d IV from day −9 until bowel function returned to normal, at which point, patients were switched to oral CsA. Every 12 hours, 0.5 g of MMF was administered orally from day −9 to day +30 after transplantation; after that time, the MMF dose was tapered to half until day 160 and discontinued thereafter.
After entering this study, patients were allowed to select postremission treatment with HRD-HSCT or chemotherapy after discussing the treatment options with their doctors. Discussions included fear of TRM, a desire for a radical cure of the disease, concerns regarding donors, and cost considerations.
Monitoring and definition of MRD
After allo-HSCT, all patients eligible for this study were monitored for minimal residual disease (MRD). The MRD target, WT1 gene expression, was determined in the diagnostic specimens by quantitative RT-PCR, as described previously.23 Abnormality was defined as > 0.6% of the WT1 gene in BM samples. The time points for monitoring MRD included +1, +2, +3, +4.5, +6, +9, and +12 months, and every 6 months after +12 month. If abnormality was detected once, monitoring was repeated 2 weeks later. MRD positivity was defined as 2 consecutive WT1 gene abnormalities in BM samples taken at 2-week intervals.
MRD-oriented intervention with DLI
MRD-positive cases were treated with donor lymphocyte infusion (DLI) unless active GVHD or signs of serious organ failure were present. For DLI, G-CSF–mobilized peripheral blood stem cells were infused instead of steady donor lymphocytes. The median numbers of CD3+ cells infused were 0.57 × 108/kg (range, 0.30-0.86). Before DLI, serious infections and GVHD were controlled. After DLI, immunosuppressive agents such as CsA or MTX were given to prevent DLI-associated GVHD. If DLI was administered within 100 days after HSCT, immunosuppressive agents were not stopped until 2-6 weeks after DLI; however, if DLI was given > 100 days after HSCT, immunosuppressive agents were discontinued for 2 weeks before DLI and then after DLI, immunosuppressive agents were given for 2-6 weeks. The initial CsA dosage was 2.5 mg/kg/d and was then adjusted to maintain a blood concentration > 100 ng/mL. The MTX dosage was 10 mg administered by IV on days +1, +4, and +8, and weekly thereafter.
End points and statistical methods
OS and DFS were measured from the start date of consolidation treatment. The end point for OS was death from any cause or, for living patients, the date of last contact. Relapse was defined as hematologic relapse. Survival functions were estimated by the Kaplan-Meier method and compared by the log-rank test. In addition, a Cox regression model was used to identify prognostic variables in patients, including: postremission treatment choice (chemotherapy or HRD-HSCT), age (> 40 years or not), sex (female or male), WBC count at diagnosis (> 50 × 109/L or not), PLT count at diagnosis (> 50 × 109/L or not), cytogenetic risk group (intermediate or high), and courses to achieving CR1 (1 course or not). End-point events for DFS included relapse and TRM in CR1. Actuarial probabilities were determined at 4 years. The independence of categoric parameters was calculated using χ2 statistics. The distribution of continuous variables was calculated using the Mann-Whitney U test.
Results
Patient characteristics
The present study enrolled 419 newly diagnosed AML patients between 15 and 60 years of age. Exclusion criteria eliminated 188 patients with standard-risk AML—61 patients with t(8;21), 30 patients with in(16), and 97 acute promyelocytic leukemia patients; 47 patients who did not achieve CR1; 45 patients who received MSD-HSCT; and 7 patients who received MUD-HSCT. There were no significant differences in patient characteristics or transplantation outcomes between the 52 patients given MSD- and MUD-HSCT and those who got HRD-HSCT (see the supplemental Appendix, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), results that are consistent with our previous studies.12,24 The remaining 132 evaluable patients were classified into either the chemotherapy-alone group (n = 74) or the HRD-HSCT group (n = 58). Among the 58 patients receiving HRD-HSCT, 6 had 1-locus–mismatched related haploidentical donors, 20 had 2-loci–mismatched donors, and 32 had 3-loci–mismatched donors. The median time to HRD-HSCT was 5 months (range, 2-7) from achievement of CR. The 2 groups (Table 1) showed no significant differences regarding sex, WBC count at diagnosis, PLT count at diagnosis, French-American-British type, or risk group. The chemotherapy group contained more patients above 30 years of age than the HRD-HSCT group (P < .0001). After HRD-HSCT, all 63 patients achieved sustained myeloid and PLT engraftment.
Only 2 MRD-positive patients received DLI for prophylaxis. DLI occurred on day 30 (patient 1) and day 140 (patient 2) after transplantation. Both received a dose of mononucleated cells of 1 × 108/kg. Patient 1 developed grade 2-3 acute GVHD and extensive chronic GVHD, relapsed at 9 months after DLI, then died of infection at 16 months after DLI. Up to the last follow-up in May 2011, patient 2 was alive without relapse with a time of 18 months.
Clinical outcomes of all patients
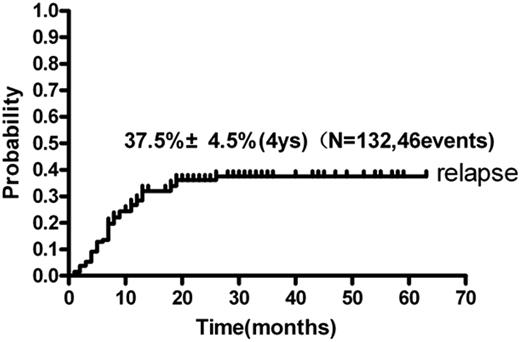
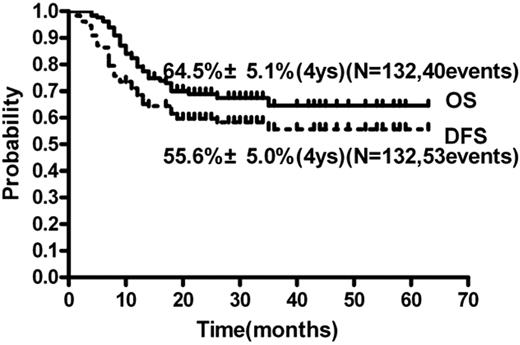
Up to the last follow-up in May 2011, the median follow-up time was 19 months (range, 4-63). Forty patients died (33 because of relapse and 7 because of TRM) and 92 patients are still alive; 46 patients experienced relapse and 79 patients are still in continuous CR1. The cumulative incidence of relapse (CIR) at 4 years was 37.5% ± 4.5% (Figure 2). The 4-year OS and DFS were 64.5% ± 5.1% and 55.6% ± 5.0% (Figure 3), respectively.
OS and DFS of all 132 patients with intermediate- and high-risk AML in CR1.
Effects of postremission therapy on clinical outcomes
Relapse.
Of 132 patients, 46 experienced relapse. Early relapses (relapses that occurred during consolidation) occurred in 4 patients after achieving CR. These patients had selected postremission treatment with HRD-HSCT or chemotherapy alone before relapse. Three patients chose chemotherapy and 1 chose transplantation. No differences in patient characteristics, including early relapses, of intermediate- and high-risk AML patients who achieved CR1 in chemotherapy and transplant groups were observed and no effects of early relapses on relapse, DFS, and OS rates were found (data not shown).
Of the 74 patients in the chemotherapy-alone group, 40 experienced relapse (including 3 extramedullary disease relapse) and received salvage chemotherapy. Two of these patients then received HRD-HSCT; 1 is still alive in CR2 and the other relapsed again after HSCT and died 3 months later. Of the 40 relapsed patients, 10 are still alive and 30 died of relapse. Among 58 patients in HRD-HSCT group, 6 experienced relapse; of these, 4 received DLI plus chemotherapy and 2 received DLI. In the DLI plus chemotherapy group, 2 patients achieved CR2 and are still alive (13 and 15 months after DLI), whereas the other 2 died of relapse (10 and 16 months after DLI). The patients who received DLI alone died without achieving CR at 3 and 4 months after relapse. Seven patients died of TRM (12.1%), including infection (n = 4), acute liver failure (n = 1), and GVHD (n = 2).
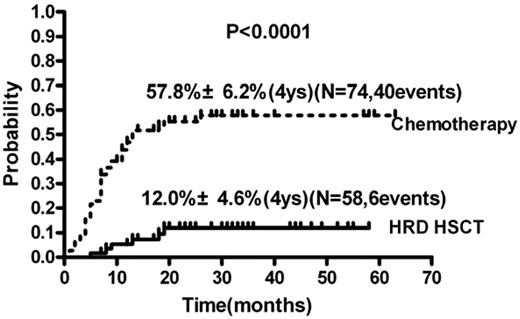
In univariate analysis, the CIR in the HRD-HSCT group was significantly lower than in the chemotherapy group (12.0% ± 4.6% vs 57.8% ± 6.2%, respectively; relative risk [RR], 0.14; 95% confidence interval [95% CI], 0.11-0.36; P < .0001; Figure 4 and Table 2). Other factors influencing relapse included WBC count at diagnosis > 50 × 109/L or not (RR = 2.07; 95% CI, 1.21-5.18; P = .013); age > 40 years or not (RR = 2.18; 95% CI, 1.26-3.97; P = .006) and number of courses to achieving CR1 of 1 or not (RR = 0.46; 95% CI, 0.22-0.77; P = .005). Other variables such as sex (female or male), PLT count at diagnosis (> 50 × 109/L or not), and cytogenetic risk group (intermediate- or high-risk) did not influence the risk of relapse.
CIR of the HRD-HSCT group and the chemotherapy-alone group of patients with intermediate- and high-risk AML.
CIR of the HRD-HSCT group and the chemotherapy-alone group of patients with intermediate- and high-risk AML.
In multivariate analyses (Table 2), the RR of relapse was 0.12 (95% CI, 0.05-0.28; P < .0001) for HRD-HSCT compared with chemotherapy and 3.08 (95% CI, 1.67-5.67; P < .0001) for high WBC count (> 50 × 109/L). Forty patients relapsed in the chemotherapy group, whereas only 6 relapsed in the HRD-HSCT group.
DFS.
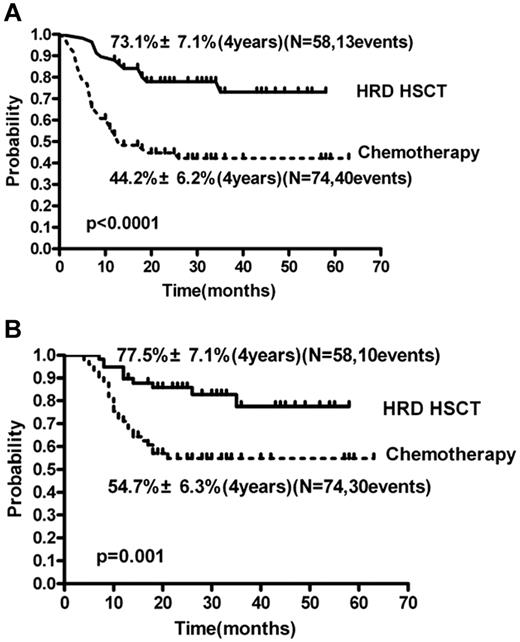
In univariate analysis, DFS of the HRD-HSCT group was significantly higher than in the chemotherapy group (73.1% ± 7.1% vs 44.2% ± 6.2%, respectively; P < .0001; RR = 0.29; 95% CI, 0.18-0.53; P < .0001; Figure 5A and Table 2). Other factors influencing DFS included WBC count at diagnosis > 50 × 109/L or not, age > 40 years or not, and number of courses to achieving CR1 of 1 or not. Other variables such as sex (female or male), PLT count at diagnosis (> 50 × 109/L or not), and cytogenetic risk group (intermediate- or high-risk) did not influence the DFS. In multivariate analysis (Table 2), the risk ratios of DFS were 0.27 (95% CI, 0.15-1.50; P < .0001) for HRD-HSCT compared with chemotherapy and 2.20 (95% CI, 1.21-3.97; P = .009) for high WBC count (> 50 × 109/L).
HRD-HSCT group and the chemotherapy-alone group of patients with intermediate- and high-risk AML. DFS (A) and OS (B).
HRD-HSCT group and the chemotherapy-alone group of patients with intermediate- and high-risk AML. DFS (A) and OS (B).
OS.
In univariate analysis, OS of the HRD-HSCT group was significantly higher than in the chemotherapy group (4 years; 77.5% ± 7.1% vs 54.7% ± 6.3%, respectively; RR = 0.32; 95% CI, 0.18-0.65; P = .001; Figure 5B and Table 2). Factors influencing OS also included WBC at diagnosis > 50 × 109/L or not, age > 40 years or not, and number of courses to achieving CR1 of 1 or not. Other variables such as sex (female or male), PLT count at diagnosis > 50 × 109/L or not, and cytogenetic risk group (intermediate- or high-risk) did not influence the OS. In multivariate analysis (Table 2), the RR of OS were 0.44 (95% CI, 0.21-0.94; P = .034) for HRD-HSCT compared with chemotherapy. Other variables such as WBC count, age, and number of courses to CR did not influence the OS.
Discussion
In this prospective study, the 4-year DFS and OS rates for the chemotherapy-alone group (44.2% ± 6.2% and 54.7% ± 6.3%, respectively) were similar to results reported by Schlenk et al.9 The 4-year DFS and OS rates (73.1% ± 7.1% and 77.5% ± 7.1%, respectively) in the haploidentical HSCT group were similar to those reported in our previous study11,12,18 and to patients who underwent MSD-HSCT and MUD-HSCT in the present study, but superior to those reported in other studies. According to a previous study,16 the event-free survival in 83 AML patients receiving haploidentical transplantation in CR ranged from 35% for those in CR2 or later to 50% for those in CR1. Another study reported outcomes of haploidentical HSCT in adults with high-risk acute leukemia25 showing a 2-year LFS in AML patients of 48% ± 10% after a CR1 transplantation and 21% ± 5% after a transplantation in ≥ CR2.25 Several factors likely contributed to our superior transplantation outcomes. First, our previous study showed that, compared with ISD transplantation (n = 36), patients with high-risk acute leukemia undergoing HRD transplantation (n = 81) had a significantly lower 2-year CIR (26% vs 49%, respectively; P = .008).19 This suggests that HRD transplantations can achieve a stronger GVL effect, which may contribute to the superior survival of AML patients with unfavorable cytogenetics. Secondly, the patients in the present study all achieved CR1 through 1-2 courses of induction chemotherapy and received 2-4 courses of consolidation therapy before transplantation. Other studies have often included AML patients in CR1 undergoing HSCT in very late phase or apparent MRD.10,26,27 In a Center for International Blood and Marrow Transplant Research analysis performed by Tallman et al,28 no clear benefit to consolidation therapy could be identified when pursuing allogenic transplantation in the MSD setting. However, in a recent study by Gupta et al relapse occurred in 37%-40% and was higher in patients receiving reduced-intensity conditioning regimens.29 These findings highlight the importance of the depth-of-remission pre-SCT, especially in recipients of the reduced-intensity conditioning group. Walter et al found that that pre-HSCT MRD is associated with increased risk of relapse and death after myeloablative HSCT for AML in first morphologic CR, even after controlling for other risk factors.30 Therefore, it is reasonable that the 2-4 courses of consolidation therapy before transplantation could lead to more depth-of-remission and contributed to the improved transplantation outcomes in the present study, although prospective, randomized trials should be explored in the future. Third, posttransplantation monitoring of WT1 transcripts by quantitative PCR, modified DLI intervention guided by MRD level, and treating relapsed patients with DLI might have contributed to our improved outcomes.23,31 Quantification of MRD after allo-HSCT is reportedly predictive of relapse, and individualized intervention can decrease relapse and improve survival in high-risk AML patients after transplantation.23,32,33
In the present study, we found that haploidentical HSCT conferred survival advantages in terms of DFS and OS compared with chemotherapy alone. Meta-analysis data and recommendations from an international expert panel suggested that, compared with chemotherapy alone, allo-HSCT from a matched related donor can achieve a better outcome for AML patients with unfavorable cytogenetics in CR1.34,35 Researchers from our center and others have also shown that HRD-HSCT can achieve outcomes comparable to those of HLA-identical sibling transplantation. In the present study, the median age of patients in the HRD-HSCT group was significantly lower than that of the chemotherapy-alone group, but multivariate analysis showed no effects of age on transplantation outcomes. Correspondingly, Dong et al suggested that outcomes of related haploidentical HSCT in hematologic malignancies are not associated with patient age.36 However, caution is essential in this interpretation because many studies37,38 have suggested that age is associated with poor transplantation outcomes. After accounting for factors known to adversely affect DFS and OS, we found significant superiority of haploidentical HSCT (relative to chemotherapy alone) in terms of DFS and OS, with 4-year DFS and OS rates of 71.3% versus 44.2% and 77.5% versus 54.7%, respectively. Haploidentical HSCT was also associated with a lower probability of relapse for intermediate- and high-risk AML patients, with 4-year CIR rates of 12.0% versus 57.8% for chemotherapy alone. Short-term observation of AML patients treated with chemotherapy alone demonstrated dramatically declining survival curves because of TRM or disease recurrence. Based on the results of this study and previous data,10,18,19 we recommend haploidentical HSCT as a viable treatment option for intermediate- and high-risk AML patients who do not have an HLA completely matched donor or who require urgent transplantation. However, it is essential to assess whether the benefit of the reduced relapse rate outweighs TRM. Comorbidity scores, such as the hematopoietic cell transplantation comorbidity index, may provide useful guidance in these decisions.39-41 Furthermore, a composite risk score that includes patient age, disease stage, time interval from diagnosis to transplantation, and donor-recipient sex combination should be considered because these variables are highly predictive of TRM, LFS, and OS in patients with AML.35,39-41
Although allo-HSCT decreases relapse in AML significantly, the high rate of TRM (15%-50%) remains the most important limiting factor to survival benefit.10,42 However, the incidence of TRM after haploidentical transplantation in the present study was 12.1%, consistent with our previous report of 19.4% in an AML group at 3 years.11 The TRM of our study was lower than that of other reports, although there is deficiency in comparability between different transplantation centers. After the GIAC transplantation protocol, flexible adjustment of GVHD and experience in treating infections and other complications may lower the TRM incidence.
For ethical and practical reasons, it was impossible to design a randomized study to compare the 2 treatment approaches for AML patients with unfavorable cytogenetics in China. To minimize this limitation, after entering this study, patients were allowed to self-select postremission treatment with HRD-HSCT or chemotherapy, although bias in the form of potential influence of what the physician says to the patient could not be completely avoided in the present study. Another limitation is the number of patients analyzed, which to a degree limits the power of the study to detect differences. Therefore, caution needs to be exercised in the interpretation of the results of this study. In addition, new molecular mutations, such as NPM, FLT3, and CEBPA, were not detected in all patients, which limits our ability to perform further subgroup analysis.34,35 Although there are a few limitations, the results of the present study confirmed that haploidentical HSCT provides significant long-term survival for patients with intermediate- and high-risk AML in CR1.
In summary, the results of the present study suggested that haploidentical HSCT in CR1 provides significant long-term survival for intermediate- and high-risk AML patients. This is the first report to compare the outcomes of treatment choice between haploidentical transplantation and chemotherapy alone in this patient group. HSCT from haploidentical related donors improved patient outcome and, because virtually all patients in need of an HSCT have an immediately available family haploidentical donor, we suggest that haploidentical HSCT should be included in the treatment algorithm as a viable option for adults with intermediate- and high-risk AML lacking a matched donor.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank San Francisco Edit (www.sfedit.net) for their assistance in editing this manuscript.
This work was supported by the Hi-Tech Research and Development Program of China (2011AA020105), the National Natural Science Foundation of China (grants 30971292 and 30800485), and the Beijing Novel program (grant 2008B05).
Authorship
Contribution: X.-J.H. served as principal investigator, designed and monitored the research, analyzed the data, and wrote the manuscript; H.-H.Z. performed the research, analyzed and interpreted the data, performed the statistical analysis, and wrote the manuscript; Y.-J.C., X.-H.Z., Y.-H.C., H.C., W.H., and Y.W. analyzed and interpreted the data; and L.-P.X., D.-H.L., B.J., Q.J., H.J., and K.-Y.L. performed the research and analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiao-Jun Huang, MD, Professor, Peking University Institute of Hematology, Peking University People's Hospital, 11 Xizhimen South Street, Beijing 100044, China; e-mail: xjhrm@medmail.com.cn.