Ever since descriptions of the various functional components of the hematopoietic stem cell niche, leukemia researchers have wondered whether leukemia stem cells (LSCs) require similar cell-extrinsic support for long-term maintenance of leukemia.2 LSCs differ from normal hematopoietic stem cells (HSCs) in that they have activation of key pathways regulating self-renewal, proliferation, and survival. Activation of these pathways is sufficient in many models for full leukemogenesis, suggesting that the LSC niche may be an attractive, but functionally redundant contributor to LSC survival. However, recent key findings point to cell-extrinsic factors in the development of leukemia,3 LSC engraftment into the hematopoietic microenvironment,4 survival after chemotherapy,5 and even the determination of leukemia phenotype,6 suggesting that components of the normal HSC niche, including osteoblastic cells, may have important roles in leukemia pathogenesis.
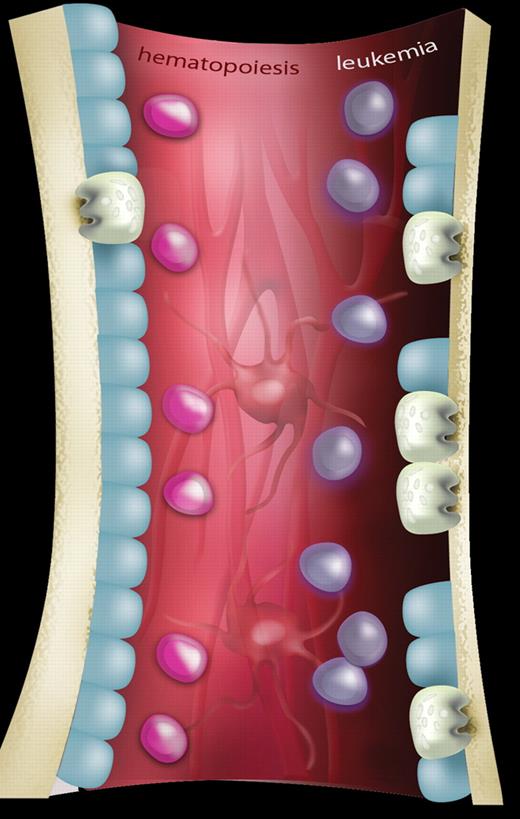
A schematic representation showing the differences in bone homeostasis between normal hematopoiesis and leukemia. In normal hematopoiesis (left side of figure), hematopoietic stem cells (HSCs; pink) are in balance with components of the hematopoietic microenvironment including osteoblastic cells (blue), osteoclasts (gray), mesenchymal cells, and vascular structures (background; red). In leukemia (right side of figure), invasion of leukemia cells (purple) results in osteopenia mediated by an expansion of osteoclasts causing increased bone resorption and a concomitant reduction of osteoblastic activity. The effect, if any, on other components of the HSC niche has yet to be determined. Artwork produced by Ms M. Kersting (QIMR).
A schematic representation showing the differences in bone homeostasis between normal hematopoiesis and leukemia. In normal hematopoiesis (left side of figure), hematopoietic stem cells (HSCs; pink) are in balance with components of the hematopoietic microenvironment including osteoblastic cells (blue), osteoclasts (gray), mesenchymal cells, and vascular structures (background; red). In leukemia (right side of figure), invasion of leukemia cells (purple) results in osteopenia mediated by an expansion of osteoclasts causing increased bone resorption and a concomitant reduction of osteoblastic activity. The effect, if any, on other components of the HSC niche has yet to be determined. Artwork produced by Ms M. Kersting (QIMR).
Frisch and colleagues now describe the somewhat unexpected finding that acute leukemia cells have direct effects on host bone formation and turnover (see figure).1 Using a retroviral bone marrow transplantation model of blast-crisis chronic myeloid leukemia, the authors observed that mice with leukemia had a reduction in bone trabeculae and thinning of cortical bone compared with wild-type, nonleukemic controls. These bony changes were mediated through both reduced bone formation and increased osteoclastic bone resorption, and were partially reversible by treatment with bisphosphonate therapy (to inhibit osteoclast function). The authors identified that leukemic mice but not normal controls had increased expression of the soluble proinflammatory cytokine CCL3 (also known as macrophage inflammatory protein-1α), a factor known to inhibit in vitro bone formation and previously implicated in myeloma-induced pathologic bone resorption.7 Finally, they were able to demonstrate increased expression of CCL3 in a significant proportion of human acute myeloid leukemia (AML) samples, suggesting that similar mechanisms may be active in patients with AML.
These findings are quite provocative and lead to many interesting and potentially clinically relevant questions. For example, is osteoporosis a common finding in acute leukemia and will screening for this provide any useful information in the acute management of the patient with newly diagnosed leukemia? In contrast to myeloma and other cancers, osteolytic lesions are not frequently encountered in patients with AML, suggesting that if these findings are present, then perhaps diffuse osteopenia, rather than discrete osteolytic lesions, is the more likely pathologic entity. Given the association with elevated CCL3 levels in AML, it is tempting to speculate that blocking this pathway may be useful in preventing bony changes. Ongoing functional work is needed to determine whether CCL3 is in fact the pathogenic molecule or whether this is one of many dysregulated chemokines in the leukemic milieu.
What, then, is the effect of these bony changes on normal host hematopoiesis? We know that osteoblastic function is an integral component of the normal HSC niche,8 and it would logically follow that impaired osteoblastic function through leukemia-secreted factors such as CCL3 may contribute to delayed hematopoietic recovery after chemotherapy. However, others have shown that a reduction in osteoblast number may not necessarily impair hematopoiesis,9 and it remains to be seen whether these leukemia-induced bony abnormalities have a negative effect on normal HSC function and recovery after chemotherapy. Along these lines, it would be important to know what, if any, effects leukemia cells have on other putative components of the HSC niche, including nestin-positive mesenchymal cells10 and vascular structures. These experiments, although technically challenging, will be valuable to further our understanding of postchemotherapy myelosuppression and potentially provide therapeutic avenues to ameliorate it.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■