To the editor:
With interest we have read the report by Chao et al,1 which is the most recent of a pioneering and impressive series of publications by the same group of researchers over the past 2 years,2-6 in which the interaction between the broadly expressed CD47 surface molecule and the myeloid inhibitory receptor SIRPα is implicated as a potential therapeutic target in a variety of hematopoietic malignancies. Chao et al use xenotransplantation models in which human NHL cells are engrafted into immunocompromised mice, and they show that lymphoma dissemination is inhibited by antibodies directed against human CD47 that block interactions with SIRPα, but not by nonblocking anti-CD47 antibodies. In similarly designed studies with AML3 and ALL5 they had already demonstrated prominent tumor cell elimination with this blocking anti-CD47 antibody, and they had also previously shown that anti-CD47 treatment synergizes with the therapeutic anti-CD20 antibody rituximab in NHL.2 Furthermore, their findings show that macrophages are essential in the process of leukemic cell elimination. They suggest that targeting of CD47-SIRPα interactions, both in the absence as well as in the presence of a cancer therapeutic antibody such as rituximab, could facilitate the eradication of tumor cells by promoting their phagocytic clearance by macrophages.6
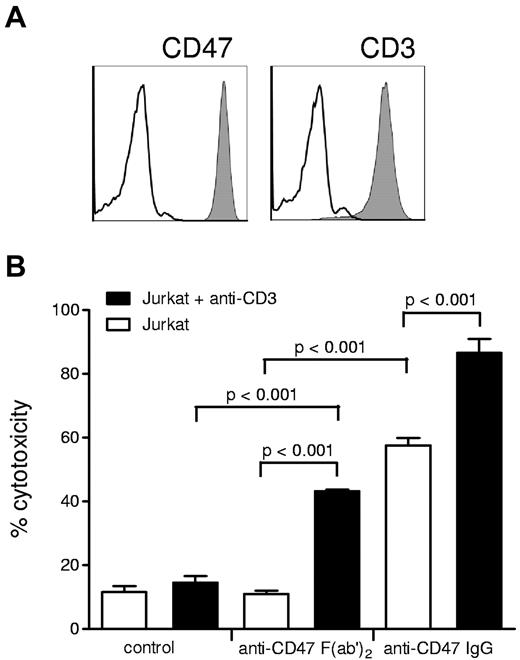
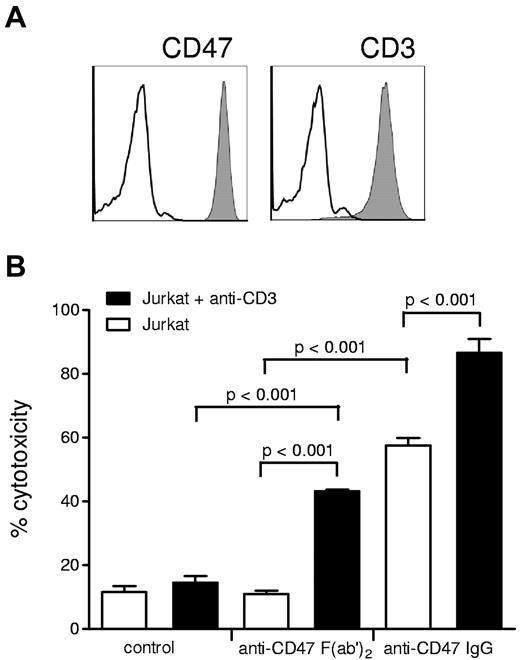
One problem that hampers a straightforward interpretation of the in vivo findings of these studies is the use of intact IgG antibodies. For instance, the anti-CD47 antibodies may not only disrupt CD47-SIRPα interactions but may instead, or at the same time, opsonize the tumor cells for antibody-dependent cellular cytotoxicity (ADCC) or antibody-dependent phagocytosis. In fact, our recent findings7 with the same monoclonal anti-CD47 antibody (B6H12) that is used in the studies described above show that intact B6H12 induces direct ADCC of neutrophils toward breast cancer cells. The same is true for ADCC by monocytes, as indicated by the cytotoxicity against Jurkat lymphoma cells (Figure 1). Clearly, direct ADCC cannot be simply excluded by using a control nonblocking anti-CD47 antibody, because this may actually have a very different capacity to induce antibody-dependent effector functions, even although it may have the same isotype. In fact, intact antibodies against CD47 might also trigger ADCC toward the healthy cells of the host, which would obviously create a highly undesirable condition. One way to really exclude this direct ADCC is to use these antibodies “stripped” of their Fc regions, that is, as B6H12 F(ab′)2 fragments. Obviously, these do not directly induce antibody effector function, but the F(ab′)2 fragments do in fact synergize with anti-CD3 antibodies against lymphoma cells (Figure 1) or with trastuzumab in the context of Her2/Neu-positive breast cancer cells.7 This suggest that at least under these in vitro conditions the targeting of CD47-SIRPα interactions only enhances tumor cell killing when an appropriate cancer therapeutic antibody such as trastuzumab or rituximab is present. Would this mechanism also apply in vivo? This is clearly something that needs to be sorted out in more detail in future experiments. However, our own findings in a fully immunocompetent syngeneic model of metastatic melanoma are promising and suggest that animals that carry a defect in the SIRPα inhibitory signaling capacity do not show enhanced elimination of tumor cells in the absence of therapeutic antibody, while in the presence of therapeutic antibody a strongly potentiated effect is observed.7
Antibody-dependent cellular cytotoxicity of human monocytes, precultured with GM-CSF for 18 hours, toward Jurkat acute T leukemia cells is enhanced by blocking anti-CD47 F(ab′)2, but intact anti-CD47 IgG induces cytotoxicity alone. Cytotoxicity was measured by the release of 51Cr from loaded target cells. (A) Surface expression of CD3 (using CLB-T3/4.2a mAb) and CD47 (using B6H12 mAb) and on Jurkat cells as evaluated by flow cytometry. (B) ADCC of human monocytes toward Jurkat cells after preincubation with mouse IgG2a T3/4.2a anti-CD3 (20 μg/mL) and/or B6H12 (10 μg/mL) anti-CD47 B6H12 F(ab′)2 or intact IgG. Values shown are means ± SD of (n ≥ 3) independent experiments. Note that intact anti-CD47 IgG alone opsonizes and induces monocyte-mediated cytotoxicity in Jurkat cells, while F(ab′)2 act only in concert with anti-CD3. P values of statistically significant differences, as determined by Student t test, are indicated.
Antibody-dependent cellular cytotoxicity of human monocytes, precultured with GM-CSF for 18 hours, toward Jurkat acute T leukemia cells is enhanced by blocking anti-CD47 F(ab′)2, but intact anti-CD47 IgG induces cytotoxicity alone. Cytotoxicity was measured by the release of 51Cr from loaded target cells. (A) Surface expression of CD3 (using CLB-T3/4.2a mAb) and CD47 (using B6H12 mAb) and on Jurkat cells as evaluated by flow cytometry. (B) ADCC of human monocytes toward Jurkat cells after preincubation with mouse IgG2a T3/4.2a anti-CD3 (20 μg/mL) and/or B6H12 (10 μg/mL) anti-CD47 B6H12 F(ab′)2 or intact IgG. Values shown are means ± SD of (n ≥ 3) independent experiments. Note that intact anti-CD47 IgG alone opsonizes and induces monocyte-mediated cytotoxicity in Jurkat cells, while F(ab′)2 act only in concert with anti-CD3. P values of statistically significant differences, as determined by Student t test, are indicated.
Another open question is whether CD47 on tumor cells could actually be efficiently targeted at all in the context of human cancer, even if it was done with the appropriate agents. The problem is that CD47 is very broadly expressed among both hematopoietic and nonhematopoietic cells, and this could seriously compromise efficient targeting of the cancer cell CD47 molecules. Instead, we propose to target SIRPα. Blocking antibodies against human SIRPα are available now7 and these would be promising agents to test further in clinical trials.
The final issue is whether the targeting of CD47 and/or SIRPα per se would not cause autoimmune disease. This subject clearly needs careful attention. In fact, there is perhaps already some comforting evidence, at least from animal experiments. First, mice that lack CD47 or have mutant SIRPα apparently do not display overt autoimmune symptoms,8,9 and second, autoimmunity is not readily induced on injection of blocking antibodies against CD47 in mice3 or against SIRPα in rats (A. van der Goes and T.K.v.d.B., unpublished data).
Collectively, we feel that the available evidence provides a strong rational basis for targeting the CD47-SIRPα interactions to potentiate the clinical efficacy of cancer therapeutic antibodies, but that more evidence is required to conclude that targeting of CD47-SIRPα alone might have beneficial effects in cancer therapy.
Authorship
Acknowledgments: Dirk Roos is gratefully acknowledged for critically reading the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Timo K. van den Berg, Sanquin Research, and Landsteiner Laboratory, Academic Medical Center, University of Amsterdam, Plesmanlaan 125, Amsterdam, 1066 CX, The Netherlands; e-mail: t.k.vandenberg@sanquin.nl.