Solulin is a soluble form of thrombomodulin that is resistant to proteolysis and oxidation. It has been shown to increase the clot lysis time in factor VIII (fVIII)–deficient plasma by an activated thrombin-activatable fibrinolysis inhibitor (TAFIa)–dependent mechanism. In the present study, blood was drawn from humans and dogs with hemophilia, and thromboelastography was used to measure tissue factor–initiated fibrin formation and tissue-plasminogen activator–induced fibrinolysis. The kinetics of TAFI and protein C activation by the thrombin-Solulin complex were determined to describe the relative extent of anticoagulation and antifibrinolysis. In severe hemophilia A, clot stability increased by > 4-fold in the presence of Solulin while minimally affecting clot lysis time. Patients receiving fVIII/fIX prophylaxis showed a similar trend of increased clot stability in the presence of Solulin. The catalytic efficiencies of TAFI and protein C activation by the thrombin-Solulin complex were determined to be 1.53 and 0.02/μM/s, respectively, explaining its preference for antifibrinolysis over anticoagulation at low concentrations. Finally, hemophilic dogs given Solulin had improved clot strength in thromboelastography assays. In conclusion, the antifibrinolytic properties of Solulin are exhibited in hemophilic human (in vitro) and dog (in vivo/ex vivo) blood at low concentrations. Our findings suggest the therapeutic utility of Solulin at a range of very low doses.
Introduction
Patients with hemophilia A have a bleeding diathesis that is usually predicted by their factor VIII (fVIII) activity level (fVIII:C).1,2 The primary form of treatment for severe hemophilia A is replacement therapy, which involves administration of recombinant or plasma-derived fVIII. FVIII can be given either on demand or by prophylaxis,3 and the amount needed can vary drastically depending on the treatment schedule and the type and severity of the bleed in the case of on-demand treatment.4
The treatment developments to date have greatly improved both mortality and morbidity for individuals with hemophilia5,6 ; however, current treatments are not 100% effective, are expensive, and are often considered inconvenient. Because single bleeding events can have devastating consequences, it is important to continue to strive for maximally effective treatments. The recent improvements in mortality and morbidity have only been observed in developed countries with the resources to fund treatment. It is currently estimated that 80% of the world's hemophilia population has little or no access to therapy7 ; therefore, the development of cost-effective alternate treatment strategies or effective factor-sparing regimes to treat bleeding is clearly necessary.
Many new and adjunctive therapeutic options have been explored, including platelet infusion,8 tranexamic acid,9 ϵ-amino caproic acid,10 molecules that block tissue factor pathway inhibitor,11,12 and a combination of phospholipid and fXa13 and fXIII.14 Solulin is a recombinant soluble analog of human thrombomodulin. Consisting of the extracellular domains of thrombomodulin, it is distinguished by several directed mutations, providing for lack of a chondroitin sulfate attachment site, resistance to exocarboxypeptidase/protease activity and to oxidation/irradiation, and, finally, abolishing the N-terminal heterogeneity arising in the wild-type sequence from 2 common signal cleavage sites.15,–17
Recently, we demonstrated that soluble thrombomodulin (Solulin) may be used to partially correct the premature lysis defect in fVIII-deficient plasma through an activated thrombin-activatable fibrinolysis inhibitor (TAFIa)–dependent mechanism,18 which supports the hypothesis that bleeding in hemophilia may be due to unregulated fibrinolysis19 in addition to the well-documented clotting defect.20 This hypothesis is also supported by a preliminary clinical study showing that ϵ-amino caproic acid, an antifibrinolytic lysine analog, may be used adjunctively with fVIII inhibitor bypass activity or activated prothrombin complex to control bleeding.10 Full-length thrombomodulin (TM) has been shown to bind tightly to thrombin,21 which prevents cleavage of fibrinogen22 and therefore fibrin formation. Furthermore, the cofactor activity of TM for thrombin-mediated protein C activation diminishes thrombin generation by proteolytically inactivating the coagulation cofactors fVa and fVIIIa.23 It is for these reasons that TM was thought to be an unlikely candidate for the treatment of bleeding in hemophilia. An important difference between Solulin and full-length TM is that Solulin has reduced affinity for thrombin, which greatly reduces its anticoagulant function but still adequately promotes TAFI activation.24 Part of the explanation lies in the absence of a chondroitin sulfate side chain in Solulin because of a mutated attachment site,15 a distinction that causes a substantial decrease in thrombin affinity.25
This is the first study using Solulin in patient samples and in dogs. We show that Solulin may be used to improve clot stability and to attenuate fibrinolysis in contact pathway–inhibited whole blood from subjects with hemophilia A or B and that the antifibrinolytic effect of Solulin at low concentrations can be rationalized by the relative kinetics of TAFI activation to protein C activation by the thrombin-Solulin complex. In addition, blood obtained from hemophilic dogs given Solulin IV was found to generate clots that are resistant to fibrinolysis, which corroborates the in vitro data obtained using human hemophilic blood.
Methods
Materials
The thrombin inhibitors D-Phe-Pro-Arg chloromethyl ketone (PPA-ck) and hirudin were purchased from Calbiochem. The TAFIa substrate anisylazoformyl arginine (AAFR; catalog number 2525) was purchased from Bachem Americas, and the activated protein C substrate S-2366 was purchased from DiaPharma Group. Recombinant human soluble thrombomodulin (Solulin) was provided by PAION Deutschland. Tissue-type plasminogen activator (Activase) was purchased from the pharmacy at KGH and protein C was purchased from Haematologic Technologies. Innovin was purchased from Siemens Healthcare Diagnostics and thrombin was prepared as described previously.26 The buffer used in all experiments was HEPES-buffered saline (20mM HEPES, 150mM NaCl, pH 7.4). All other reagents were of analytical quality.
Subjects
A total of 17 patients with hemophilia (15 with hemophilia A and 2 with hemophilia B) were recruited. All subjects gave informed, written consent before sample collection in accordance with the Declaration of Helsinki (St. Thomas' Hospital ethics approval number 10/H0805/023). The 2 subjects with hemophilia B had 5.8 and 1.6 IU/dL of fIX at baseline and 12 of 15 hemophilia A subjects had a clinically severe phenotype (with fVIII:C < 1.2 IU/dL at the time of baseline sampling). One hemophilia A subject (patient 11) had a higher than expected factor level due to noncompliance with the requested washout period. Patients were free of inhibitors at the time of blood collection and their demographics and clinical details are presented in Table 1. Three patients had abnormally low platelet counts; 2 of these consistently had platelet counts ranging from 110-150 and the third had a low platelet count on this occasion but all previous and subsequent counts were within the normal range. Subjects 9 and 17 were included in analysis despite having a low platelet count because their counts were only marginally low and thromboelastography parameters were similar to other patients enrolled. In addition, 2 patients had a history of hepatitis C, which was treated and resolved.
Thromboelastography
Blood was drawn from human subjects with hemophilia A or B into 3.2% trisodium citrate and 30 μg/mL of corn trypsin inhibitor (9 parts blood and 1 part anticoagulant) before and, for select subjects, at various times after fVIII or fIX administration, which was given for clinical purposes. Within 30 minutes, the blood (260 μL) was titrated with Solulin (20 μL, 0-100nM) and induced to clot with Innovin (1:17 000) and CaCl2 (5mM excess; 20 μL). Tissue-plasminogen activator (20 μL, 1nM) was included to induce fibrinolysis (final concentrations). Rotational thromboelastometry (ROTEM; Pentapharm) measurements were used to determine the clot lysis time (CT; in minutes) and maximum clot firmness (MCF; in millimeters). In addition, the area under the elasticity curve (AUEC; in millimeters per minute) was used to characterize and quantify the stability of fibrin.9,14
Kinetics of protein C and TAFI activation by thrombin-Solulin
Solulin (25nM) and thrombin (0.5nM) were incubated in HEPES-buffered saline at room temperature in wells of a transparent 96-well plate. TAFI or protein C (0-2μM) was added to a final volume of 40 μL to start the reaction. Each experiment was conducted in the presence of 2.5mM CaCl2. After a 10-minute reaction time, 160 μL of a solution containing 150μM AAFR and 1.25μM PPA-ck was added to the TAFI series and 160 μL of a solution containing 375μM S-2366 and 3.125 antithrombin units/mL of hirudin was added to the protein C series. In these experiments, PPA-ck and hirudin were used to quench the respective reactions and the small substrates AAFR and S-2366 were used in conjunction with standards to determine the amount of TAFI or protein C activated over the 10-minute experimental time frame. The rates of TAFI and protein C activation were determined using these methods and subsequently analyzed by nonlinear regression to the Michaelis-Menten model of enzyme kinetics.
Animals
Dogs with hemophilia A were bred and maintained at Queen's University, Kingston, ON. All procedures were in compliance with the institutional animal care committee and the Canadian Council for Animal Care. Blood from 4 dogs with severe hemophilia A27 was collected before and at regular intervals after IV Solulin administration. Solulin was given by bolus injection IV in a volume of 3 mL (3 dogs: 5 or 10 μg/kg; 1 dog: 500 μg/kg). The dosing of Solulin is described in relative terms with a “low” dose being 5 or 10 μg/kg and a “high” dose being 500 μg/kg. The blood was subsequently analyzed by thromboelastography (see next section). A parallel sample was used for the determination of plasma concentration of Solulin using an ELISA assay validated for dog plasma.
Ex vivo thromboelastography using hemophilic dog blood
Citrated blood (9 parts blood and 1 part 3.8% trisodium citrate) was obtained from hemophilic dogs before and after IV administration of Solulin. The blood was subjected to thromboelastography using methods similar to those described in “Thromboelastography.” Briefly, 340 μL of whole blood was added to channels of a Hemoscope TEG 5000 (Hemonetics) containing a 20-μL solution of Innovin (1/15 000 dilution) and CaCl2 (5mM excess) to induce coagulation and tissue-type plasminogen activator (1nM) to induce fibrinolysis (final concentrations). After mixing thoroughly, the pin was seated and coagulation and fibrinolysis were monitored continuously. Because of equipment limitations, the maximum amplitude (MA; maximum clot strength) was used to describe the efficacy of Solulin in stabilizing fibrin.
Statistics
Each Solulin dosage was grouped and between-group comparisons were conducted using ANOVA and the Tukey HSD test. Data were presented as means ± SD. For all statistical analyses, P < .05 was considered significant.
Results
Effect of Solulin on clot stability and CLT in patients with severe hemophilia A
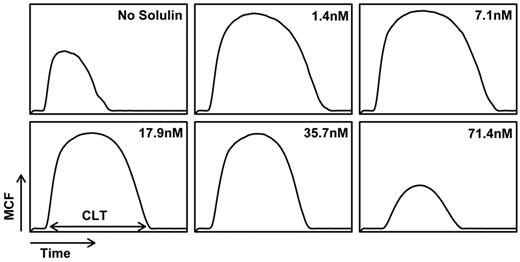
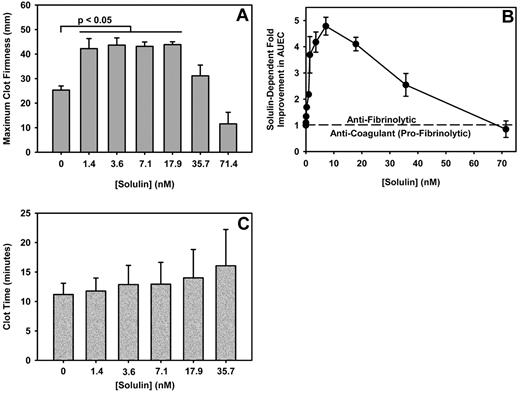
Using ROTEM, the effect of Solulin on fibrin viscoelasticity was monitored using thromboelastography. At Solulin concentrations ranging from 1.4 to 35.7nM, ROTEM parameters generally improved compared with the control containing no Solulin (Figure 1). The MCF and CLT increased dramatically over this range of Solulin concentrations, which resulted in an associated increase in the AUEC. In the absence of Solulin, the MCF of fibrin formed from severe hemophilic blood was 25.3 ± 4.5 mm (Figure 2A). Solulin concentrations ranging from 1.4-17.9nM significantly increased the MCF (P < .01) by an average of 1.7-fold (43.2 ± 6.6 mm). At 35.7nM Solulin, a nominal increase in MCF was still observed (P = .12); however, the MCF decreased from its maximal level (31.1 ± 11.6 mm). This reduction was likely because of increased protein C activation, as may be conjectured from the virtually complete suppression of thrombin generation observed when Solulin was studied ex vivo in healthy volunteers.28 Like MCF, the AUEC was also significantly increased in severe hemophilia in the presence of Solulin (1.4-17.9nM; P < .05), due in part to an increase in MCF, but also to an increased CLT. Figure 2B shows that the AUEC was increased by approximately 4-fold compared with the no-Solulin controls (16.8 ± 4.6 × 104 vs 4.1 ± 0.8 × 104 mm/min, respectively). Consistent with the concurrent (nominal) increase in MCF, 35.7nM Solulin still improved the AUEC (by 2.5-fold, P < .05) compared with controls, but the level was lower (10.2 ± 4.7 × 104 mm/min) than that achieved with 1.4-17.9nM Solulin. At 71.4nM Solulin a decrease in both the MCF (11.6 ± 12.5 mm) and AUEC (3.4 ± 3.3 × 104 mm/min) was observed, which is best explained by the anticoagulant properties of Solulin prevailing over its antifibrinolytic properties. To demonstrate that Solulin minimally affects the CLT in hemophilic whole blood, CLT data were analyzed for the concentrations of Solulin that improved the MCF and AUEC (ie, 1.4-35.7nM; Figure 2C). In the absence of Solulin, the CLT was 11.2 ± 1.9 minutes and increased slightly (by < 16%; P > .05) in the presence of 1.4-7.1nM Solulin (from 11.8 ± 2.2 to 12.9 ± 3.7 minutes). The increase in CLT did not reach the level of statistical significance at Solulin concentrations less than 40nM.
Solulin improves fibrin viscoelasticity. At low concentrations (1.4-35.7nM), Solulin increases the MCF and CLT in human hemophilic blood. Increases in MCF and CLT are accompanied by an increase in the AUEC, which is used as a measure of clot stability or resistance to fibrinolysis. At high concentrations of Solulin (71.4nM), the MCF, CLT, and AUEC are reduced compared with the no-Solulin control. These data are representative of those obtained when Solulin and hemophilic blood (< 1% fVIII:C) are titrated.
Solulin improves fibrin viscoelasticity. At low concentrations (1.4-35.7nM), Solulin increases the MCF and CLT in human hemophilic blood. Increases in MCF and CLT are accompanied by an increase in the AUEC, which is used as a measure of clot stability or resistance to fibrinolysis. At high concentrations of Solulin (71.4nM), the MCF, CLT, and AUEC are reduced compared with the no-Solulin control. These data are representative of those obtained when Solulin and hemophilic blood (< 1% fVIII:C) are titrated.
Solulin increases the MCF and AUEC in human hemophilic blood with minimal effect on CLT. (A) The MCF increased from 25.3 ± 4.5 mm to 43.2 ± 6.6 mm in the presence of 1.4-17.9nM Solulin (P < .01). At higher concentrations, the MCF decreased from peak values. (B) In severe hemophilia A, the AUEC is increased by approximately 4-fold in the presence of Solulin due in part to an increase in MCF but also to an increased CLT. (C) Solulin marginally increases the CLT in hemophilia A blood. At the lowest concentrations of Solulin (< 7.1nM), the CLT increased marginally from 11.2 ± 1.9 to 12.9 ± 3.7 minutes. The increase in CLT with Solulin was not statistically significant even at 35.7nM (P = .07).
Solulin increases the MCF and AUEC in human hemophilic blood with minimal effect on CLT. (A) The MCF increased from 25.3 ± 4.5 mm to 43.2 ± 6.6 mm in the presence of 1.4-17.9nM Solulin (P < .01). At higher concentrations, the MCF decreased from peak values. (B) In severe hemophilia A, the AUEC is increased by approximately 4-fold in the presence of Solulin due in part to an increase in MCF but also to an increased CLT. (C) Solulin marginally increases the CLT in hemophilia A blood. At the lowest concentrations of Solulin (< 7.1nM), the CLT increased marginally from 11.2 ± 1.9 to 12.9 ± 3.7 minutes. The increase in CLT with Solulin was not statistically significant even at 35.7nM (P = .07).
Effect of fVIII or fIX on the Solulin-dependent improvement of clot stability
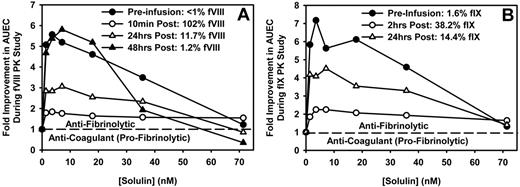
Three of the patients enrolled (2 with hemophilia A and 1 with hemophilia B) were studied longitudinally after an approximately 5-day washout period. The first sample in this series of experiments was collected just before the patient received fVIII or fIX for clinical purposes. This baseline sample and other samples collected after factor infusion were induced to clot and lyse, as described in “Thromboelastography,” and analyzed by thromboelastography to elucidate how fVIII or fIX influences the antifibrinolytic effect of Solulin. Figure 3A shows that before fVIII infusion (< 1% fVIII), Solulin, at low concentrations (< 17.1nM), improved clot stability (as shown by the AUEC) by up to 6-fold in this particular subject's blood. In absolute values, the increment was similar after administration of fVIII (102% fVIII:C). However, as clot stability increased after fVIII:C administration (3.7 just before and 11.6 × 104 mm/min after fVIII:C, no Solulin), the improvement by Solulin relative to the no-Solulin control (from 11.6 × 104 to 21.3 × 104 mm/min) was only 1.84-fold. As the time from fVIII infusion increased, the plasma concentration of fVIII decreased and the (relative) > 5.5-fold improvement in AUEC at 3.6nM Solulin was restored. Therefore, the absolute AUEC values at Solulin concentrations ranging from 1.4-17.1nM were similar regardless of fVIII:C (range, 13.7 × 104 to 21.3 × 104 mm/min), whereas the relative increases were most pronounced at low fVIII:C.
Solulin improves clot stability (AUEC) in human hemophilic blood over a range of fVIII or fIX concentrations (exemplified in single patients). Solulin improvement of clot stability plateaued at concentration ranging from 1.4-7.1nM regardless of fVIII:C (A) or fIX:C (B). Shown is the relative improvement over fVIII:C or fIX:C alone. As the reference AUEC increased after factor infusion, the relative improvement in clot strength by Solulin decreased. The absolute Solulin-dependent increase in AUEC can be calculated by multiplying the relative improvement in AUEC by the AUEC in the absence of Solulin (fVIII or fIX as a percentage, AUEC in units of 104 millimeters per minute; panel A, < 1, 3.67; 102, 11.59; 11.7, 6.31; 1.2, and 2.98; panel B, 1.6, 2.33; 38.2, 7.32; 14.4, and 4.17).
Solulin improves clot stability (AUEC) in human hemophilic blood over a range of fVIII or fIX concentrations (exemplified in single patients). Solulin improvement of clot stability plateaued at concentration ranging from 1.4-7.1nM regardless of fVIII:C (A) or fIX:C (B). Shown is the relative improvement over fVIII:C or fIX:C alone. As the reference AUEC increased after factor infusion, the relative improvement in clot strength by Solulin decreased. The absolute Solulin-dependent increase in AUEC can be calculated by multiplying the relative improvement in AUEC by the AUEC in the absence of Solulin (fVIII or fIX as a percentage, AUEC in units of 104 millimeters per minute; panel A, < 1, 3.67; 102, 11.59; 11.7, 6.31; 1.2, and 2.98; panel B, 1.6, 2.33; 38.2, 7.32; 14.4, and 4.17).
The hemophilia B subject, who was studied longitudinally, demonstrated a Solulin-dependent increase in the AUEC before fIX infusion (Figure 3B), which was similar to that observed in the hemophilia A subject (Figure 3A). Two hours after fIX infusion, the fIX:C was 38.2% and the AUEC in the absence of Solulin had increased from 2.3 × 104 to 7.3 × 104 mm/min. As with the hemophilia A subject, the absolute AUEC values at a given Solulin concentration were comparable throughout, whereas the increases relative to the values without Solulin were almost exclusively a function of the latter. For example, before fIX infusion (ie, 1.6% fIX), 3.6nM Solulin caused a 7.3-fold increase in AUEC, from 2.3 × 104 to 16.7 × 104 mm/min. This AUEC value remained virtually unchanged at 16.3 × 104 mm/min 2 hours later, when fIX:C had reached 38.2%, whereas the relative increase was only 2.23-fold (16.3/7.3). As the fIX:C level dropped further to 14.4%, the relative improvement in the AUEC approached the pre-infusion level.
Kinetics of protein C and TAFI activation by the thrombin-Solulin complex
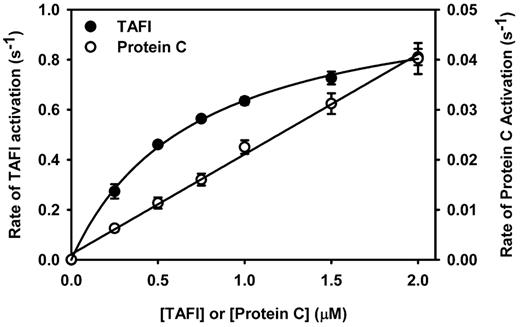
To rationalize the preference for antifibrinolysis over anticoagulation at low concentrations of Solulin, the kinetics of TAFI and protein C activation by the thrombin-Solulin complex were determined and compared. Figure 4 shows that the thrombin-Solulin complex activated TAFI much more efficiently than it did protein C. As described previously,29 TAFI activation by the thrombin-Solulin complex can be described using the Michaelis-Menten model. Using the Michaelis-Menten model to fit the data, a Km of 0.71μM and a kcat of 1.09/s were determined, which implies a catalytic efficiency of 1.53/μM/s. The slope derived from the protein C activation data provides a reasonable estimate of the catalytic efficiency of the reaction. The catalytic efficiency of protein C activation by the thrombin-Solulin complex was 0.02/μM/s, which is 76-fold less that that observed for TAFI activation by the same complex.
Kinetics of TAFI and protein C activation by the thrombin-Solulin complex in HEPES-buffered saline. TAFI activation (●) was fit by nonlinear regression to the Michaelis-Menten model of enzyme kinetics, yielding a Km of 0.71μM and a kcat of 1.09/s, implying a catalytic efficiency of 1.53/μM/s. The kinetic parameters Km and kcat cannot be determined for protein C activation by the thrombin-Solulin complex (○); however, the slope provides a reasonable estimate of the catalytic efficiency (kcat/Km = 0.02/μM/s).
Kinetics of TAFI and protein C activation by the thrombin-Solulin complex in HEPES-buffered saline. TAFI activation (●) was fit by nonlinear regression to the Michaelis-Menten model of enzyme kinetics, yielding a Km of 0.71μM and a kcat of 1.09/s, implying a catalytic efficiency of 1.53/μM/s. The kinetic parameters Km and kcat cannot be determined for protein C activation by the thrombin-Solulin complex (○); however, the slope provides a reasonable estimate of the catalytic efficiency (kcat/Km = 0.02/μM/s).
Ex vivo effect of Solulin on clot stability in blood from hemophilic dogs
Dogs given a low dose of Solulin (5 or 10 μg/kg) generally showed improvement in their maximal clot strength after Solulin administration (Figure 5A-C). Because of time and equipment constraints, clot strength (MA) was used to describe the effect of Solulin on fibrin stabilization. Figure 2A and B show that clot firmness (MA) and clot stability (AUEC) follow very similar trends. Dog 1 (Figure 2A) had a 40-mm improvement in maximal clot strength (amplitude) 2 hours after Solulin administration, at an actual plasma concentration of Solulin of 0.8nM Solulin. Dog 2 (Figure 2B) showed the best improvement in clot strength. A marginal increase in clot strength was observed after 0.5 and 2 hours (1.1 and 0.87nM Solulin, respectively). During the period from 8-72 hours (0.9-0.1nM Solulin), there was a sustained improvement in clot strength compared with the baseline value (20-35 mm improvement). Dog 3 (Figure 2C) also exhibited an improvement in clot strength during the 72-hour period after Solulin administration (up to a 25-mm improvement at 72 hours; 0.16nM Solulin). The dog given a high dose of Solulin (500 μg/kg; Figure 5D) was actually the first experiment in the series, the dose being selected from the range of doses studied in human volunteers.28 The dog showed a decrease in clot strength during the first 24 hours after receiving Solulin. As the concentration of Solulin decreased to approximately 40nM at the 48-hour time point, a marginal increase in clot strength was observed. The clot strength improved further at the 72-hour time point, when the Solulin concentration had decreased to approximately 22nM, indicating that lower doses were appropriate for further exploration.
Clot firmness is increased in blood from hemophilic dogs given Solulin at low doses (5 or 10 μg/mL). Thromboelastography shows that the individual response to low-dose Solulin varied among the 3 dogs enrolled (panels A, B, and C show data for doses of 5, 10, and 10 μg/kg, respectively). The most dramatic improvement was observed in dog 2 (B), in which clot strength (amplitude) was increased throughout the 72-hour monitoring period. Dog 4 (D), which was given a high dose of Solulin (500 μg/kg) showed decreased clot strength (amplitude) during the first 24 hours after receiving Solulin. As the concentration of Solulin decreased in dog 4, an improvement in clot strength was observed, suggesting an overdose.
Clot firmness is increased in blood from hemophilic dogs given Solulin at low doses (5 or 10 μg/mL). Thromboelastography shows that the individual response to low-dose Solulin varied among the 3 dogs enrolled (panels A, B, and C show data for doses of 5, 10, and 10 μg/kg, respectively). The most dramatic improvement was observed in dog 2 (B), in which clot strength (amplitude) was increased throughout the 72-hour monitoring period. Dog 4 (D), which was given a high dose of Solulin (500 μg/kg) showed decreased clot strength (amplitude) during the first 24 hours after receiving Solulin. As the concentration of Solulin decreased in dog 4, an improvement in clot strength was observed, suggesting an overdose.
Discussion
In the present study, we report proof of principle that Solulin may be used to improve clot strength and stability in hemophilic whole blood. We demonstrate that in vitro Solulin improves clot stability in severe hemophilia A and B, and that the Solulin-dependent improvement in clot strength is maintained throughout the peaks and troughs of factor replacement therapy. As could be predicted, such improvement is less marked when high factor concentrations already provide for an acceptable clot firmness, and more marked when such factor effects are lower because of lower factor concentrations. We also show herein that at low concentrations (30 pM to 35nM), the antifibrinolytic properties of Solulin are more pronounced than anticoagulant properties, which are rationalized by the relative efficiencies of TAFI and protein C activation by the thrombin-Solulin complex. Finally, our preliminary data suggest that Solulin administered to dogs with severe hemophilia improves ex vivo thromboelastography parameters in a manner similar to that observed in vitro with blood from severe hemophilia human subjects.
The relative kinetics of TAFI and protein C activation by the thrombin-Solulin complex provides a biochemical rationale for the preference for antifibrinolysis at low Solulin concentrations with minimal anticoagulation. The kcat (1.1/s) and Km (0.71μM) of TAFI activation by the thrombin-Solulin complex suggest that TAFI is a much better substrate for the complex than is protein C, which has a linear Michaelis plot (ie, a high Km). Given the potency of TAFIa in down-regulating fibrinolysis,18,24 only a very small fraction is needed to maximally attenuate fibrinolysis. Once this level of TAFIa is achieved, increasing the Solulin concentration would have no additional effect on fibrinolysis, but would result in increased protein C activation. The impact of this shift from a state of antifibrinolysis to that of antifibrinolysis combined with anticoagulation is evident when examining the effect of Solulin on clot stability. In the present study, at 3.6nM Solulin, the AUEC was increased by 4.5-fold, whereas at 71.4nM Solulin, the AUEC was decreased compared with the no-Solulin control (Figure 2B). Our data demonstrate that a higher concentration of Solulin compared with full-length thrombomodulin (rabbit lung) is required30 to shift the hemostatic balance from antifibrinolytic to anticoagulant (> 35nM compared with approximately 15nM).
The advantages of using an agent such as Solulin to stabilize fibrin are intriguing, but do not replace the need for factor replacement or bypassing products, and therefore one envisages that Solulin would be most efficacious as an adjunctive pro-hemostatic agent. The key features of Solulin that make it an interesting option for the treatment for hemophilia is its long half-life28 and wide effective dose range (Figure 2). With a 15- to 30-hour half-life and effective dose range estimated to range from the sub-nanomolar to approximately 40nM, Solulin could potentially be administered on a weekly basis and provide the basis for a factor-sparing regime that would cut costs and make therapy more widely available. However, before proceeding to advanced trials, safety concerns stemming from the anticoagulant properties of Solulin must be addressed. The effect of overdosing with Solulin was evident herein in dog 4 (Figure 5D), in which a greater than 35nM Solulin decreased clot strength, implying enhanced anticoagulation. To minimize bleeding risk, certain Solulin mutants may be useful in increasing the safety profile of the potential drug. Both oxidized M388-Solulin and F376A-Solulin retain most of their cofactor activity for TAFI activation, but only a small fraction of their cofactor activity for protein C activation.31 Using these constructs may increase the therapeutic window of Solulin by making it safer at higher doses.
Fibrin formation and deposition is essential for normal hemostasis. Fibrin forms as a direct consequence of thrombin generation, and because this is impaired in hemophilia, so too is fibrin formation.32 Solulin is a novel compound with potential for the treatment of hemophilia and, perhaps more importantly, illustrates a novel paradigm in which fibrin formation and maintenance is targeted in addition to enhancing thrombin generation. A new paradigm in which fibrin is targeted may result in the development of therapeutics that decrease the incidence of bleeding at trough fVIII:C and make therapies more accessible to those in developing countries.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kirsten Christiansen for technical assistance and the staff at the Hemophilia Center, St Thomas' Hospital, for help in recruiting patients.
This work was supported by the National Institutes of Health (grant HL46703 project 4 to M.E.N.) and by the Heart and Stroke Foundation of Canada (grant T5575 to M.E.N.). The animal studies were sponsored by PAION Deutschland GmbH. For a portion of this study, J.H.F. was funded by a Heart and Stroke doctoral fellowship and a Queen's University doctoral field research travel award. D.L. is the recipient of a Canada Research Chair in Molecular Hemostasis.
National Institutes of Health
Authorship
Contribution: J.H.F. coordinated the study, designed and executed the experiments, analyzed the data, and wrote the manuscript; K.-U.P. coordinated the study, analyzed the data, and contributed to writing the manuscript; C.J.R. performed the experiments, recruited the patients, and edited the manuscript; L.H. and S.P. performed the animal experiments; D.L. coordinated the animal experiments and edited the manuscript; M.E.N. designed the experiments; and B.S. designed the experiments, coordinated the human in vitro studies, and edited the manuscript.
Conflict-of-interest disclosure: J.H.F. has received research funding and speaker honoraria from PAION Deutschland GmbH. K.-U.P. is an employee of PAION Deutschland GmbH, which has a commercial interest in Solulin. B.S. has participated in advisory board meetings for PAION Deutschland GmbH and received a consultancy fee. The remaining authors declare no competing financial interests.
M.E.N. is deceased.
Correspondence: Jonathan H. Foley, Department of Biochemistry, University of Vermont, 208 S Park Dr, CRF, Rm 248, Colchester, VT 05446; e-mail: jonathan.foley@uvm.edu.