In this issue of Blood, Kochenderfer and colleagues update their results of a phase 1 trial describing the infusion of autologous T cells rendered specific for CD19.1 Their data build on the safety, feasibility, and efficacy regarding adoptive transfer of genetically modified T cells expressing a chimeric antigen receptor (CAR).
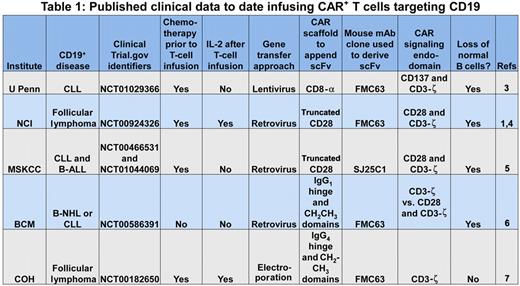
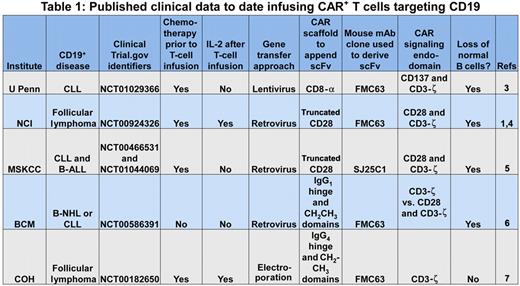
The prototypical CAR initially pioneered by Dr Zelig Eshhar2 appropriates the scFv region of a monoclonal antibody (mAb) to recognize a cell-surface tumor-associated antigen independent of the major histocompatibility complex (MHC), and one or more signaling molecules to activate genetically modified T cells for killing, proliferation, and cytokine production. This report by Kochenderfer et al supports the premise that an introduced CAR can redirect T-cell specificity for CD19 and the promise that clinical-grade CAR+ T cells can be successfully applied to cancer gene therapy.1 Their data join other published observations demonstrating that CD19-specific CAR+ T cells can be adoptively transferred to treat patients with refractory B-lineage lymphomas and chronic lymphocytic leukemia (see Table 11,3-7 ). These trials typically employ chemotherapy regimens before adoptive immunotherapy to promote the lymphopenia-induced proliferation and thus persistence of the infused T cells. This approach to combining chemotherapy with T-cell therapy, so successfully used for T-cell treatment of advanced melanoma, likely benefits the survival of CAR+ T cells, but it is associated with increased toxicity and can complicate attribution of the observed antitumor effects.
U Penn indicates University of Pennsylvania; NCI, National Cancer Institute; MSKCC, Memorial Sloan-Kettering Cancer Center; BCM, Baylor College of Medicine; COH, City of Hope; CLL, chronic lymphocytic leukemia; B-ALL, B acute lymphoblastic leukemia; and B-NHL, B non-Hodgkin lymphoma.
U Penn indicates University of Pennsylvania; NCI, National Cancer Institute; MSKCC, Memorial Sloan-Kettering Cancer Center; BCM, Baylor College of Medicine; COH, City of Hope; CLL, chronic lymphocytic leukemia; B-ALL, B acute lymphoblastic leukemia; and B-NHL, B non-Hodgkin lymphoma.
To define the potency of infused CAR+ T cells for B-cell neoplasms, investigators have recognized that genetically modified T cells do not distinguish between CD19 expressed on normal and malignant B cells. Thus, the prolonged absence of normal B cells, following recovery of hematopoiesis after chemotherapy, serves as a biomarker for the therapeutic potential of the adoptively transferred T cells. All of the second-generation CAR designs8 resulted in sustained depletion of normal numbers of CD19+ B cells (see Table 1). In other words, all the CARs that were designed to activate T cells via a chimeric CD28 or CD137 endodomain, in concert with CD3-ζ, were also able to target and kill normal B cells. Furthermore, the number of genetically modified T cells administered did not appear to correlate with elimination of (normal and malignant) B cells and the scaffolding motifs used to suspend the CAR from the cell surface and the type of recombinant retrovirus used to transduce T cells also did not appear to adversely affect potency.
The loss of normal B cells may have minimal clinical impact to the recipient if their antibody levels are maintained by infusion of intravenous immunoglobulin. Of greater importance to the patient is whether the infused T cells produce an antitumor effect. In the current study, autologous T cells expressing a second-generation CAR (signaling through chimeric CD28 and CD3-ζ) were co-infused with high-dose intravenous IL-2 in lymphodepleted recipients and this resulted in remissions in 6 of 7 evaluable patients with advanced B-cell malignancies. These observations extend the original case report4 as we now learn that the initial CD19+ lymphoma patient relapsed but entered into a partial remission after redosing with CAR+ T cells. These data support the clinical observations by the teams at the University of Pennsylvania and Memorial Sloan-Kettering Cancer Center who also document antitumor effects stemming from infusions of patient-derived CD19-specific T cells after chemotherapy.3,5 Together with observations that infusing GD2-specific and CD20-specific T cells can result in antitumor effects,9,10 it appears convincing that CAR+ T cells can treat a subset of malignancies that are otherwise refractory to conventional therapies.
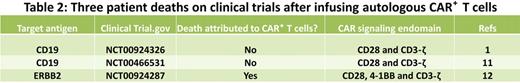
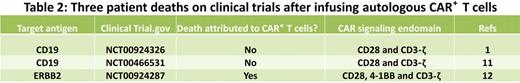
The adoptive transfer of CAR+ T cells can, however, also lead to toxicities. There have been patient deaths on 3 clinical trials (see Table 21,11,12 ), but importantly, the 2 deaths occurring in patients with B-cell malignancies were not attributed to the infusion of CD19-specific CAR+ T cells. Heavily pretreated cancer patients enrolled on CAR+ T-cell studies do appear susceptible to acute, but reversible, toxicities that are attributable to the genetically modified T cells that are related to “T-cell engraftment,” and somewhat captured under the term “cytokine release syndrome” in the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4 (http://evs.nci.nih.gov/ftp1/CTCAE/About.html). The T-cell therapy field is indebted to the high-quality and in-depth correlative studies undertaken by these trials' investigators to dissect these adverse events that appear to be heralded, and likely correlated in at least 2 trials,1,5 by rises in serum cytokine levels, and in particular elevations in interferon-γ. These toxicities can occur late after the T-cell infusion and it appears that attention to supportive care, and if necessary the systemic administration of corticosteroids, can control these adverse events. Maneuvers that may help maintain patient safety include splitting a T-cell dose over 2 or more days as well as preventing synchronous activation of CD19-specific CAR+ T cells by intra- and interpatient dose-escalation schemes and reducing antigen load (eg, by administering Rituximab for CD19+CD20+ malignancies7 ).
Human trials will continue to be needed to assess and re-assess the therapeutic potential of CAR+ T cells. There remain unresolved questions regarding CAR design (eg, second-generation CARs using chimeric CD28 versus CD137 endodomains versus third-generation CARs employing both) and determining the optimal delivery system (eg, viral versus nonviral and retrovirus versus lentivirus). In addition, there remain issues of: (1) reducing acute toxicities; (2) the role of naive and memory CAR+ T cells contributing to antitumor effects; (3) evidence of replication senescence among manufactured T cells; (4) the role for administration of exogenous cytokines; (5) the role of B cells to serve as an in vivo cellular substrate to enhance T-cell persistence; (6) whether T cells can be administered in states of minimal disease; and (7) whether to ablate CAR+ T cells to restore normal B-cell number and function. Trials are now under way in many countries infusing CAR+ T cells targeting a variety of tumor-associated antigens in an attempt to address all these issues.
In the United States, the development and implementation of CAR+ T cells has been largely supported by funding from the National Institutes of Health and private foundations with protocols conceived by and undertaken at not-for-profit academic centers. As the current study highlights, CAR+ T cells may soon be poised to achieve widespread clinical impact and there may soon be opportunities to advance CAR+ T-cell therapies by for-profit entities.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health