Abstract
Clinical and laboratory features of 642 consecutive Chinese subjects with primary myelofibrosis (PMF) were analyzed and compared with those of 1054 predominately white subjects with PMF. Chinese subjects were significantly younger, fewer had constitutional symptoms, and fewer had a palpable spleen or liver. Anemia, in contrast, was significantly more common in Chinese as was an increased white blood cell count and low platelet count. The reason for these differences is unclear, but it does not seem to be correlated with delayed diagnosis. A small but significantly increased proportion of Chinese had the JAK2V617F mutation but no difference in the frequency of haplotypes associated with PMF in whites. Survival of Chinese with PMF was also significantly longer than that of whites with PMF. We found commonly used staging systems for PMF such as the International Prognostic Scoring System and the Dynamic International Prognostic Scoring System were suboptimal predictors of survival in Chinese with PMF, and we developed a revised prognostic score that should help in comparison of data between studies of PMF in different populations and planning of clinical trials.
Introduction
Primary myelofibrosis (PMF) is a clonal hematopoietic stem cell disorder classified as a myeloproliferative neoplasm.1-5 Most reports of clinical and laboratory features of persons with PMF are from whites from Europe and North America and include few Asians. These white-orientated datasets have been used to develop prognostic indices such as the International Prognostic Scoring System (IPSS),6 the Dynamic IPSS (DIPSS),7 and the DIPSS-Plus.8
Some data suggest important differences between Asians and whites with hematologic neoplasms. For example, chronic lymphocytic leukemia is common among whites but rare in Asians.9 Others reported correlations between DNA haplotypes and risk of myeloproliferative neoplasms.10 Based on these considerations, one might expect differences in PMF between whites and Chinese.
We analyzed data from 642 consecutive subjects with PMF from 1 Chinese center. We focused on 2 issues: (1) clinical and laboratory features, and (2) prognostic variables. Results of these analyses were compared with those reported for predominately white subjects (1054) with PMF. We found significant differences in both spheres, with important clinical implications.
Methods
Study sample and data sources
The study was approved by the ethics committees of the Institute of Hematology, Chinese Academy of Medical Sciences, and Peking Union Medical College, according to guidelines of the Declaration of Helsinki. Consecutive subjects with PMF diagnosed January 1986 to December 2009 at the Institute of Hematology and Blood Disease Hospital, Chinese Academy of Medical Sciences were enrolled. Diagnosis of PMF was based on World Health Organization criteria4 and were similar to findings reported previously.6 Anemia was defined as a hemoglobin value less than 100 g/L. Spleen and liver enlargement were based on physical exam. Chromosome analyses used R- or G-banding of unstimulated bone marrow cells after 24-hour culture. Cytogenetic abnormalities were classified according to International System for Human Cytogenetic Nomenclature (1995). JAK2V617F analysis was performed on bone marrow cells as described previously.11
The Institute of Hematology and Blood Disease Hospital (Tianjin, China) is the only national specialized hematology hospital in China offering diagnosis and treatment to persons countrywide. Subjects in this study were from 22 provinces. Medical management varied and was usually based on disease features in each subject. Our approach in asymptomatic subjects was typically “wait-and-see” until disease progression. Symptomatic persons typically received, single-drug oral therapy, mainly with hydroxyurea, but also with busulfan, 6-mercaptopurine, and thioguanine. Other therapies included androgenic steroids, erythropoiesis-stimulating drugs, prednisone, interferon-α, and thalidomide. Six subjects receiving allotransplant were excluded. In China, RBC transfusions are typically given only for a hemoglobin levels less than 60 g/L.
Statistical analysis
Descriptive and statistical analyses were based on parameters ascertained at diagnosis. Statistical procedures used were conventional and all data were analyzed by using SPSS13.0 software (Version 13.0; SPSS). P values were 2-tailed, and statistical significance was set at P less than .05. Comparison between categorical variables was performed by χ2 statistics. Comparison of continuous variables between different categories was performed by the Mann-Whitney U test. Survival was measured from day 0 to death from any cause or last known follow-up and estimated using the Kaplan-Meier method. Log-rank test was used to compare survival data. A Cox proportional hazards regression model was used for multivariate analyses. Data were censored on June 30, 2011, or at date of last subject contact. Median follow-up of surviving subjects is 24 months (range, 1-347 months).
Results
Cohort characteristics
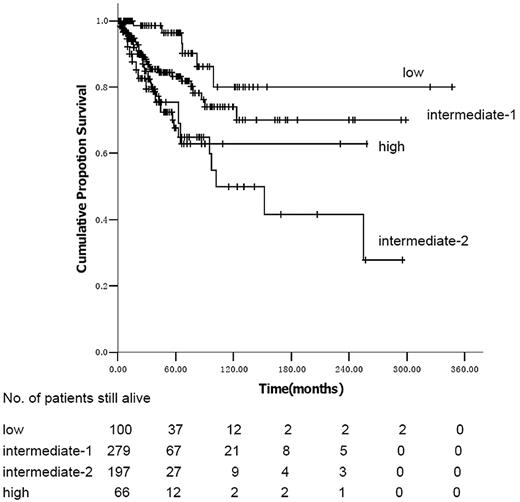
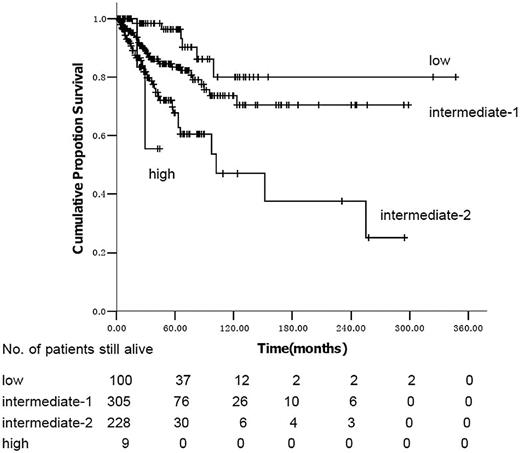
We identified 642 consecutive subjects with PMF between 1986 and 2009. Median age was 53 y (range, 14-85 years); 358 subjects (56%) were male. Clinical and laboratory variables are outlined in Table 1. The JAK2V617F mutation was identified in 159 (67%) of 238 consecutively tested subjects: 132 of 186 subjects tested had normal cytogenetics; 54 had 1 or more cytogenetic abnormalities; 17 had +(8) only, 6 had del(20q) only, 5 had a complex karyotype (≥ 3 cytogenetic abnormalities), and 26 had cytogenetic abnormalities that individually occurred in less than 5 subjects. IPSS risk score was low in 100 subjects (16%), intermediate-1 in 279 (43%), intermediate-2 in 197 (37%), and high in 66 (10%). DIPSS risk was low in 100 subjects (10%), intermediate-1 in 305 (48%), intermediate-2 in 228 (36%), and high in 9 (1%).
There is no difference of JAK2V617F mutation between the splenomegaly group (63/104, 63%) and the no splenomegaly group (94/134, 70%; P = .214). Numbers of immature myeloid cells in blood are significantly higher (P = .003) in the palpable splenomegaly group (0.41 [0-31] × 109/L) than in the nonpalpable splenomegaly group (0.16 [0-4.34] × 109/L). Numbers of nucleated red blood cells in the blood are also significantly higher in the splenomegaly cohort (0.16 × 109/L [range, 0-4.34 × 109/L]) than the no splenomegaly cohort (0.03 × 109/L [range, 0-107 × 109/L]; P = .005). Mean corpuscular volume of the anemia cohort was 91.2 fL (range, 77.6-130.6 fL), and value significantly higher than the no anemia cohort, 89.1 fL (range, 77.3-122.7 fL; P < .001). Ferritin levels in the anemia cohort (337 μg/L; range, 22-1847 μg/L) were also significantly higher than those of the no anemia cohort, 156 μg/L (range, 21-1628 μg/L; P < .001).
Clinical and laboratory features of Chinese compared with those reported for predominately whites with PMF
We compared clinical and laboratory features of Chinese subjects with those of 1054 predominately white subjects (Table 2) reported by the International Working Group for Myelofibrosis Research and Treatment.6 Chinese subjects were significantly younger: 18% were 65 years of age or older versus 45% in predominately Western subjects (P < .001). Also, fewer Chinese subjects had constitutional symptoms (21% vs 27%; P = .006). Splenomegaly was one half as common (45% vs 89%; P < .001) and hepatomegaly was one third as common (17% vs 50%; P < .001).
Complete blood count values were also different between Chinese and predominately white subjects with PMF (Table 2). Anemia was twice as common in Chinese as predominately Western subjects (67% vs 35%; P < .001). WBC count (> 25 × 109/L) was also more common (15% vs 10%; P = .002). In contrast, platelet count was lower in Chinese subjects (46% had platelets < 100 × 109/L vs 18% for predominately Western subjects; P < .001). Conversely, platelet counts of more than 400 × 109/L were present in 16% of Chinese subjects versus 31% of predominately Western subjects (P < .001). Blood blasts more than 1% were also less common in Chinese subjects (18% vs 37%; P < .001). JAK2V617F was slightly but significantly more common in Chinese (67% vs 59%; P = .04; Table 2). Sex distribution and cytogenetics were similar between the cohorts.
Survivors and prognostic scores of PMF in China
The differences in clinical and laboratory variables resulted in differences in the distribution of IPSS and DIPSS scores in Chinese and predominately white subjects (Table 3). Using the IPSS criteria, Chinese subjects were less often low-risk than predominately white subjects because of the increased proportion with hemoglobin values less than 100/L (16% vs 22%; P < .001). However, they were also less likely to be high risk because of higher platelet levels (10% vs 21%; P < .001). Chinese subjects were more likely to be in the low- and intermediate-1 risk cohorts than predominately white subjects (59% vs 42%; P < .001). The converse was so using the DIPSS criteria, where 37% of Chinese subjects were in the intermediate-2 and high-risk cohorts versus 17% of predominately white subjects (P < .001).
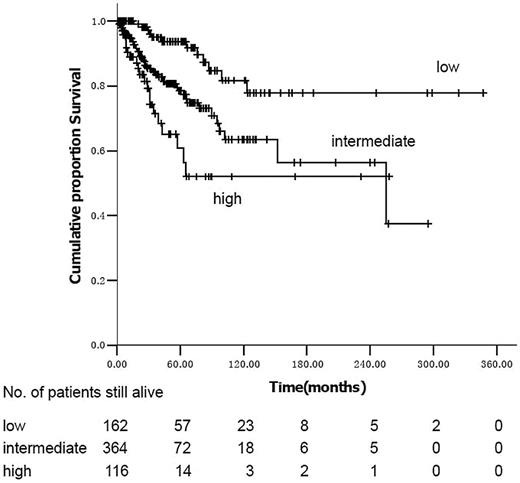
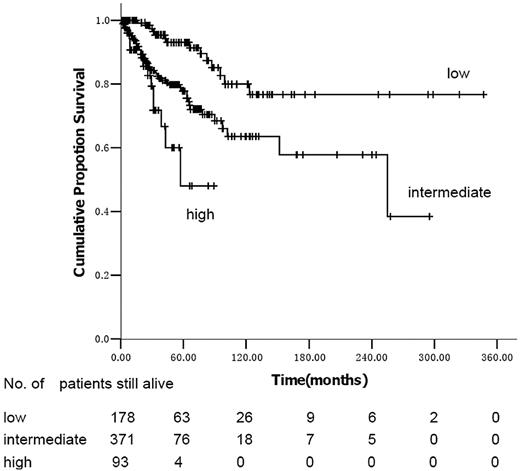
Using the IPSS model, the survival curves of intermediate-2 and high-risk subjects are not significantly different (P = .97; Figure 1). Median survival was not reached in low- and intermediate-1–risk subjects and was 102 months (95% confidence interval [CI], 30-174 months) in intermediate-2 subjects using the DIPSS risk categories (Figure 2). In univariate analyses, variables significantly correlated with survival were male sex (hazard ratio [HR] = 1.74; 95% CI, 1.12-2.70; P = .013), age more than 65 years (HR = 2.19; 95% CI, 1.38-3.48; P = .001), hemoglobin less than 100 g/L (HR = 3.89; 95% CI, 2.25-6.72; P < .001), platelets less than 100 × 109/L (HR = 1.95; 95% CI, 1.27-2.99; P = .002), WBCs less than 10 × 109/L (HR = 1.71; 95% CI, 1.08-2.70; P = .020), no spleen enlargement to palpation (HR = 2.06; 95% CI, 1.32-3.22; P = .001), RBC transfusion dependence (HR = 2.42; 95% CI, 1.53-3.83; P < .001), IPSS, and DIPSS. When the 186 cytogenetic evaluable patients were categorized according to the previously reported cytogenetic classification system for PMF,8 34 (18%) were unfavorable karyotype and 152 (82%) were favorable karyotype; the overall survival was 255 months (range, 1-258 months) and not reached, respectively (P = .625). Multivariate analysis identified platelets less than 100 × 109/L, no splenomegaly to palpation, and IPSS or DIPSS as independent risk factors for survival (Tables 4 and 5). We assigned integer score weights close to the corresponding HR (Tables 4 and 5) and merged consecutive score values into 3 risk categories: low risk (score 0-1), intermediate risk (score 2-3), and high risk (score 4-5). The modified DIPSS is better than the modified IPSS in predicting survival of Chinese subjects with PMF (Figures 3 and 4). Median survival was not reached in low-risk subjects (N = 178), was 21.3 years (95% CI, 16.4-29.5 years) in intermediate-risk subjects, and was 4.8 years (95% CI, 4.0-6.1 years) in high-risk subjects.
Kaplan-Meier estimate of survival in PMF according to the modified IPSS.
Kaplan-Meier estimate of survival in PMF according to the modified DIPSS.
Discussion
Our data show clinical and laboratory features of Chinese subjects in China with PMF at diagnosis differ substantially from those of predominately white subjects with PMF in Western countries. Chinese subjects at diagnosis were younger; were less likely to have constitutional symptoms; less frequently had palpable spleens and livers; and were more likely to have anemia, low platelet counts, and low blood blast levels but higher WBC counts than predominately White subjects with PMF.
We considered several reasons that might account for these differences. First, were we dealing with a different disease? This is unlikely. Diagnostic criteria for PMF between Chinese and predominately white subjects with PMF were similar. Bone marrow biopsy slides from our subjects were reviewed by 2 pathologists using World Health Organization criteria with high diagnostic concordance.
We also considered whether differences in health care access might account for different clinical and laboratory features. However, the constellation of features we found in Chinese patients with PMF does not support this notion. For example, although a higher proportion of Chinese subjects had a hemoglobin level less than 100 g/L at diagnosis compared with predominately Western subjects, suggesting delayed diagnosis, Chinese subjects were significantly younger than predominantly white subjects and a lower proportion had palpable spleens and livers. These findings are somewhat surprising considering possible less access to health care and frequency of routine blood testing in China. These findings are inconsistent with a hypothesis of delayed ascertainment of PMF in Chinese subjects as the cause of different clinical and laboratory features. The relatively similar frequencies of JAK2V617F suggest that the Chinese cohort was not contaminated with a high proportion of undiagnosed cases of polycythemia vera because almost all these subjects would have had the JAK2V617F mutation.
We also considered whether the frequency of α-thalassemia, iron deficiency, or both between Chinese and predominately white subjects might account for differences in disease phenotype, directly or indirectly, such as by affecting anemia severity. Molecular testing for α-thalassemia was not done in our cohort and not reported for the predominately white cohort. Values for mean corpuscular volume and for ferritin were significantly higher in Chinese subjects with anemia than those without anemia. These values are not reported for the predominately white cohort, so no direct comparison is possible. Interestingly, a substantial proportion of subjects in the predominately white cohort are from Mediterranean countries where α-thalassemia is also common, as it is among Han Chinese. Consequently, we think differences in the background incidence of α-thalassemia are unlikely to explain the large difference in anemia frequency at diagnosis between Chinese and predominately white subjects with PMF, but this conclusion requires confirmation.
We also considered possible cultural differences. For example, might Chinese subjects be more willing to accept anemia and constitutional symptoms than predominately white subjects? If so, we would expect a higher proportion with a palpable spleen, liver, or both and a higher proportion with an increased WBC count. However, we found the contrary.
Survival of the 2 cohorts was also different. Median survival of Chinese subjects was 79 months versus 69 months in predominately white subjects.6 This survival difference cannot be accounted for by differences in the time of diagnosis because Chinese subjects had a different distribution of the DIPSS score that should accommodate differences in time of ascertainment.
These data suggest there are fundamental biologic and clinical differences between Chinese and predominately white subjects with PMF. This outcome is not entirely surprising. Many well-defined diseases differ between races and ethnic groups. For example, myelodysplastic syndrome has different clinical features in Asians and whites.12-16 The same is so for β-thalassemia and other hematologic disorders.17,18 The most striking example is chronic lymphocytic leukemia that is a common leukemia in whites but rare in Asians.9 These differences probably reflect the different genetic backgrounds on which an acquired genetic disorder such as PMF occurs.
Cross et al19 reported the rs12340895 and rs10974944 haplotypes in whites were correlated with a significantly increased risk of PMF. Also, they reported preferential occurrence of the JAK2V617F on this haplotype in heterozygotes. We found similar rs12340895 and rs10974944 haplotype frequencies in normal Chinese as those reported in whites. We also found a similar frequency of these haplotypes in Chinese and predominately whites with PMF as well as preferential occurrence of the JAK2V617F on these haplotypes (Z.J.X., manuscript in preparation). Consequently, a difference in the frequency of these haplotypes does not seem to explain the different biologic and clinical features between Chinese and predominately Western subjects with PMF.
Not surprisingly, prognostic variables for Chinese and predominately white subjects with PMF overlap but are not identical. For example, fewer Chinese were in intermediate-1– and high-risk cohorts using the IPSS and DIPSS prognostic indices than predominately white subjects. This is reflected in longer survival among Chinese with PMF.
Because the IPSS and DIPSS prognostic indices are derived from predominately Western subjects, it may not operate optimally in Chinese with PMF. For example, only 1% of Chinese are classified as high risk in the DIPSS. Consequently, we developed a prognostic score to predict survival of Chinese subjects. This score divides Chinese subjects into 3 groups and can be used physicians for counseling and for the comparison of and design of clinical trials. Whether it should be used for Chinese subjects with PMF in Western countries requires testing.
Our study raises the interesting question of why Chinese with PMF residing in China should differ so substantially from predominantly white subjects with PMF residing in Europe and North America. Studies are underway to address this question, including analyses of Chinese persons with PMF living in Western countries.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work is supported in part by National Natural Science funds (81070403), Tianjin Key Natural Science funds (08JCZDJC19200), the Tianjin Key Technology R&D Program (09ZCZDSF03800), a Key International S&T Cooperation project (2010DFB30270), National Science and Technology Major project (2011ZX09302-007-04), and a National Public Health Grand Research Foundation (No. 201202017).
Authorship
Contribution: Z.J.X. designed the research, was the principal investigator, and took primary responsibility for the paper; Z.J.X. and R.P.G. wrote the paper; Z.F.X., Y.Z., T.J.Q., T.J.Z., L.L., S.Q.Q., and Z.J.X. recruited the patients; and H.S.C. and P.H.Z. reviewed the pathology.
Conflict-of-interest disclosure: R.P.G. is a part-time employee of Celgene Corp., Summit, NJ. The remaining authors declare no competing financial interests.
Correspondence: Zhijian J. Xiao, Department of Clinical Hematology, Institute of Hematology, Chinese Academy of Medical Sciences, 288 Nanjing Rd, Tianjin 300020, China; e-mail: zjxiao@hotmail.com.