Abstract
Allogeneic hematopoietic cell transplantation in multiple myeloma is limited by prior reports of high treatment-related mortality. We analyzed outcomes after allogeneic hematopoietic cell transplantation for multiple myeloma in 1207 recipients in 3 cohorts based on the year of transplantation: 1989-1994 (n = 343), 1995-2000 (n = 376), and 2001-2005 (n = 488). The most recent cohort was significantly older (53% > 50 years) and had more recipients after prior autotransplantation. Use of unrelated donors, reduced-intensity conditioning and the blood cell grafts increased over time. Rates of acute graft-versus-host (GVHD) were similar, but chronic GVHD rates were highest in the most recent cohort. Overall survival (OS) at 1-year increased over time, reflecting a decrease in treatment-related mortality, but 5-year relapse rates increased from 39% (95% confidence interval [CI], 33%-44%) in 1989-1994 to 58% (95% CI, 51%-64%; P < .001) in the 2001-2005 cohort. Projected 5-year progression-free survival and OS are 14% (95% CI, 9%-20%) and 29% (95% CI, 23%-35%), respectively, in the latest cohort. Increasing age, longer interval from diagnosis to transplantation, and unrelated donor grafts adversely affected OS in multivariate analysis. Survival at 5 years for subjects with none, 1, 2, or 3 of these risk factors were 41% (range, 36%-47%), 32% (range, 27%-37%), 25% (range, 19%-31%), and 3% (range, 0%-11%), respectively (P < .0001).
Introduction
Autologous hematopoietic stem cell transplantation (auto-HCT) is standard therapy for eligible patients with multiple myeloma (MM), and MM is the most common indication for auto-HCT in North America.1,2 In contrast, allogeneic hematopoietic cell transplantation (allo-HCT) is less commonly used because of high treatment-related mortality (TRM).3 Most reports on the utility of allogeneic approaches have come from smaller series or from transplantation registries.4-7 Higher rates of molecular responses after allo-HCT compared with auto-HCT and a potential graft-versus-myeloma effect have been considered significant benefits for this modality.8-11
Despite higher TRM, there has been continued interest in the role of allo-HCT because of the inability to cure patients with currently available therapies, improved ability to identify those with high-risk myeloma, advances in supportive care, and increasing experience with nonmyeloablative/reduced-intensity (NMA/RIC) conditioning. Data from the European Group for Blood and Marrow Transplantation suggest that TRM with allo-HCT decreased from 46% at 2 years for transplantations performed from 1983-1993 to 30% for transplantations done from 1994-1998.12 A study from Seattle also highlighted the reduction in TRM in recent years.13 Recent trials have attempted to compare the NMA/RIC allogeneic approach to tandem auto-HCT, especially in the subgroup of patients with high-risk disease.14,15 Prospective trials of RIC regimens also suggest lower TRM.16-19
It is not clear how these changes have affected outcomes after allo-HCT in MM. Data reported to the Center for International Blood and Marrow Transplantation Research (CIBMTR) offers a unique resource to study these trends. We analyzed the trends in patient demographics, disease characteristics, and rates of GVHD, TRM, progression-free survival (PFS), and overall survival (OS) among patients undergoing allo-HCT for MM from 1989 to 2005.
Methods
Patient population
Recipients of allo-HC transplants for MM between 1989 and 2005 reported to the CIBMTR were included in this study. The CIBMTR is the research affiliation of the International Bone Marrow Transplant Registry and the National Marrow Donor Program (NMDP). Established in 2004, it receives data from > 500 transplantation centers worldwide on consecutive allo-HCTs and auto-HCTs. Data are submitted to the Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis, where computerized checks for discrepancies, physicians' review of submitted data, and on-site audits of participating centers ensure data quality. The CIBMTR collects both Transplant Essential Data and Comprehensive Report Form data before transplantation, 100 days and 6 months after transplantation, and annually thereafter. Observational studies conducted by the CIBMTR are performed with approval of the institutional review boards of the National Marrow Donor Program and the Medical College of Wisconsin.
Statistical analysis
Patient-, disease-, and transplantation-related variables of allo-HC transplant recipients for MM were analyzed in 3 cohorts based on their year of transplantation (1989-1994, 1995-2000, and 2001-2004). Cohorts were compared with the χ2 statistic for categorical variables and the Kruskal-Wallis test for continuous variables. Probability of PFS and OS were calculated with the Kaplan-Meier estimator, with the variance estimated by Greenwood formula. Values for other endpoints were generated with the use of cumulative incidence estimates to accommodate competing risks. Comparison of survival curves was done with the log-rank test.
Potential prognostic factors for survival were evaluated in a multivariate analysis with the use of the Cox proportional hazards regression. The variables considered in the multivariate analysis were age at transplantation (continuous), time from diagnosis to transplantation (≤ 24 months vs > 24 months), type of donor (HLA-identical sibling vs other related vs unrelated donor), donor recipient sex match, prior auto-HCT (no vs yes), and conditioning regimen (myeloablative vs NMA/RIC). A stepwise model selection approach at a .05 significance level was used to identify all significant risk factors. Each step of model building contained the main effect for year of transplantation (1989-1994 vs 1995-2000 vs 2001- 2005). In the model, the assumption of proportional hazards was tested for each variable, and factors violating the proportionality assumption were adjusted by stratification. All variables considered in the multivariate analysis satisfied the proportionality assumption. The potential interactions between the main effect and all other significant risk factors were tested. Adjusted probabilities of OS were generated from the final Cox models. All computations were made with the statistical package SAS Version 9 (SAS Institute). All P values are 2-sided.
Results
Patient characteristics
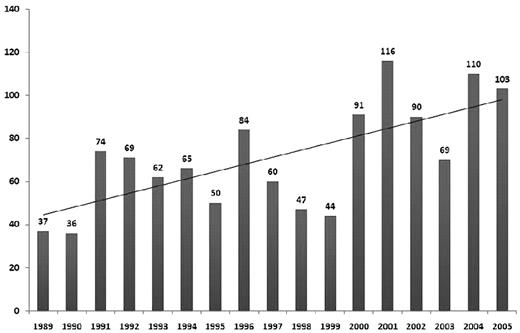
The study population consisted of 1207 patients, analyzed in 3 cohorts according to the year of transplantation: 1989-1994 (n = 343; 28%), 1995-2000 (n = 376; 31%), and 2001-2005 (n = 488; 41%). Patients were reported from 121 transplantation centers, including 59 centers from Europe that contributed 359 patients (30%). Cutoff date for follow-up for the data presented was June 30, 2008. Follow-up completeness index for the overall study was 97%. Patient characteristics are detailed in Table 1. The numbers of allo-HCT for MM reported to CIBMTR over the years are shown in Figure 1.
Number of allo-HCTs for MM reported to the CIBMTR during 1989 and 2005 annually with trend line.
Number of allo-HCTs for MM reported to the CIBMTR during 1989 and 2005 annually with trend line.
The median age at transplantation for the entire group was 47 years (range, 22-70 years), and 732 (61%) of the patients were male. Age at transplantation increased in the later cohorts with a median of 51 years in the recent cohort (2001-2005) compared with 44 years for the early group (1989-1994). The proportions of patients > 50 years were 12%, 36%, and 53% during the 3 consecutive time periods, respectively. Similarly, 13% (n = 63) of the patients in the most recent time period were > 60 years compared with none of the patients in the earliest cohort. The progressive increase in the overall numbers of patients undergoing allo-HCT over the 3 time periods is completely accounted for by patients > 50 years. Patients were also reported from more centers in the last time period: 121 compared with 84 during the first time period. The Karnofsky performance score at transplantation was similar across the 3 groups, with 61% having a score of ≥ 90%. The majority of the patients (80%) in the dataset were lacking cytogenetic information at diagnosis because such testing was not in routine clinical practice for most of the time period under study.
Disease characteristics
Baseline disease characteristics were not significantly different between the time periods, but this comparison was hampered by a significant amount of missing data, especially in the earlier cohorts. This reflects incomplete reporting as well as changes in staging system and response criteria for MM. The distribution of heavy chain type and the proportion of patients with light chain or nonsecretory myeloma were similar across the groups. The distribution of international staging system stage could not be compared because almost all patients had their disease diagnosed before the advent of the staging system. Overall, the proportion of patients with sensitive disease at the time of transplantation was similar in the latter 2 groups, whereas this information was not available for most of the patients in the earliest group. The median time from diagnosis to transplantation was 14 months for the entire group and was similar across the 3 groups (Table 1). Given the lack of data about the intent to perform allo-HCT in a planned “upfront” fashion or as salvage after relapse, we considered allo-HCT within 12 months of diagnosis as a surrogate marker for early allo-HCT. The proportion of patients undergoing an allo-HCT within 12 months of diagnosis was similar across the 3 groups with 37%-49% receiving an allo-HCT earlier in the course of their disease. The number of lines of therapy before allo-HCT was not available for most of the patients in the earliest time period, thereby limiting comparison.
Transplantation-related characteristics
A clear shift was observed toward the use of RIC or NMA regimens in the most recent cohort (Table 2). Although cyclophosphamide with total body irradiation (Cy-TBI) and busulfan with Cy-conditioning regimens constituted 82% of all transplant-conditioning regimens in the first group, they accounted for only 8% in the most recent cohort. The most commonly used conditioning regimens in the recent group included melphalan ≤ 150 mg/m2, low-dose (200 cGy) TBI, and fludarabine with TBI, accounting for 50% of the patients. A significantly higher proportion of patients (64%) in the most recent cohort had an auto-HCT before the allo-HCT compared with 3% and 23%, respectively, in the first and second cohort. The increase in the use of tandem auto-allo HCT was clearly evident with 50% of the recipients in the most recent cohort receiving allo-HC transplant within 6 months of the auto-HCT compared with 20% in the previous 2 cohorts combined.
Increasing use of peripheral blood stem cell (PBSC) grafts was observed with 88% of the transplantations in the recent cohort that used PBSC grafts compared with < 1% in the first cohort. There was increasing use of unrelated donors with 32% of the recent cohort receiving unrelated transplants compared with 5% in the first cohort. GVHD prophylaxis continues to be primarily cyclosporine (CsA) based with 81% and 64% in the first and the latest cohorts receiving a CsA-based prophylaxis regimen. However, the proportion of patients getting methotrexate in combination with CsA decreased over the time period from 69% in the first cohort to 21% in the third cohort. Use of T-cell depletion declined over this time period, 17%, 21%, and 3% for the 3 consecutive cohorts, respectively. There was increasing use of antithymocyte globulin either as part of conditioning or for GVHD prophylaxis from 2% to 19% over the time periods, probably reflecting increased use of RIC regimens and unrelated donors.
Outcomes after transplantation
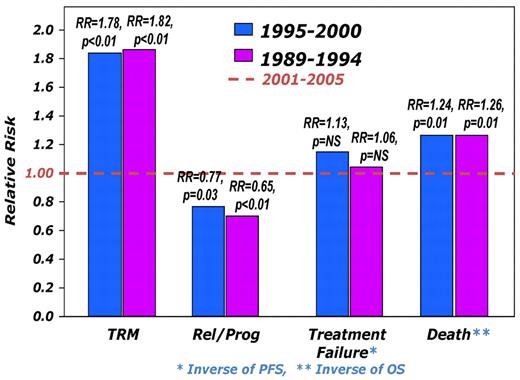
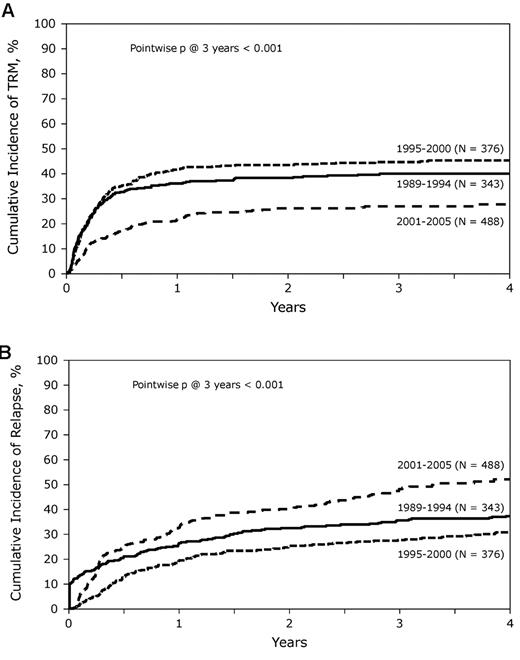
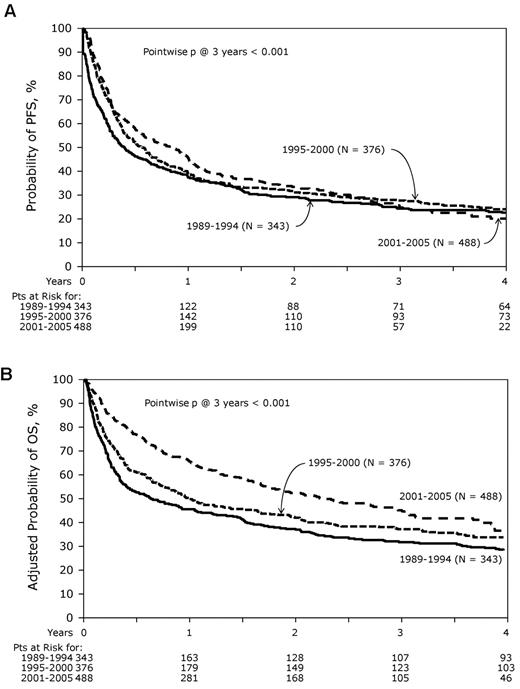
At the time of analysis, 411 patients were alive with a median follow-up for the surviving patients of 62 months (range, 3-204 months). Incidence of acute GVHD (grade II-IV) was similar across the 3 cohorts (Table 3). However, the incidence of chronic GVHD was significantly higher in the most recent cohort, with one-half of the patients surviving beyond 5 years having chronic GHVD. Sixty-five percent of these patients had extensive chronic GVHD, and 46% had moderate-to-severe chronic GVHD. There was a significant decrease in TRM during this time period with patients in the most recent cohort enjoying a 45% reduction in the risk of TRM compared with the 2 earlier time periods (P < .01), whereas no difference was seen between the 2 earlier cohorts (Figures 2 and 3A). In contrast, the risk of relapse was highest in the most recent cohort with a cumulative incidence of relapse of 58% at 5 years compared with 39% at 5 years in the earliest group and 32% for the intermediate cohort (Figures 2 and 3B). However, PFS was comparable across the 3 groups, reflecting the opposing effects of increased risk of progression and decreased TRM (Figure 4A). Although OS at 1 year was superior in the most recent cohort (63% vs 49% vs 50%; P < .001; Figure 2), at 3 and 5 years OS was similar across all 3 cohorts (Figure 4B). The causes of death were similar across the groups with the exception of deaths because of progressive disease being lowest in the 1995-2000 group (Table 4). The distribution of early and late mortality after transplantation is detailed in Table 5. We also estimated the OS from the time of relapse after allo-HCT, and this was comparable between the 3 groups.
Relative risks (RRs) of various outcomes after allo-HCT during the 3 time periods. The risk is expressed relative to that seen during the most recent period (2001-2005), which is assumed to have a RR of 1.
Relative risks (RRs) of various outcomes after allo-HCT during the 3 time periods. The risk is expressed relative to that seen during the most recent period (2001-2005), which is assumed to have a RR of 1.
Cumulative incidence of TRM and relapse. (A) Cumulative incidence of TRM during the 3 time periods studied. (B) Similarly, cumulative incidence of relapse over time, comparing the results from the 3 time periods.
Cumulative incidence of TRM and relapse. (A) Cumulative incidence of TRM during the 3 time periods studied. (B) Similarly, cumulative incidence of relapse over time, comparing the results from the 3 time periods.
PFS and OS. (A) PFS and (B) OS from diagnosis. The curves are compared with the log-rank test. Patients at risk of the event are as shown below the graph.
PFS and OS. (A) PFS and (B) OS from diagnosis. The curves are compared with the log-rank test. Patients at risk of the event are as shown below the graph.
Factors affecting survival
We specifically examined the effect of various baseline and transplant-related characteristics on OS after allo-HCT (Table 6). Compared with the initial cohort there was decreased risk of death over time. Other factors associated with improved survival were younger age, HLA-identical sibling donor, and transplantation within 24 months from diagnosis. Compared with the early cohorts, the most recent cohort had a significantly improved survival within the first 6 months after transplantation, but survival beyond 6 months after transplantation was similar across the cohorts. Other factors considered were not found to be significant in the multivariate analysis. With the use of only those with available data, we examined the effect of cytogenetic abnormality on outcome and did not find any significant difference between those with or without abnormalities on conventional cytogenetic analysis. However, significant baseline imbalances and missing data in the earlier cohorts require caution in interpreting the direct comparisons.
Risk model
The modifiable risk factors (unrelated donor grafts, increasing age [> 47 years] and longer interval [> 24 months] from diagnosis to allo-HCT) identified in multivariate analysis were used to generate a risk model for survival after allo-HCT (Figure 5). Five-year survival rates for subjects with none, 1, 2, or 3 of the risk factors were 41% (range, 36%-47%), 32% (range, 27%-37%), 25% (range, 19%-31%), and 3% (range, 0%-11%), respectively (P < .0001).
Probability of survival for risk groups with 0, 1, 2, or 3 risk factors identified from multivariate analyses.
Probability of survival for risk groups with 0, 1, 2, or 3 risk factors identified from multivariate analyses.
Discussion
Studies have suggested a near doubling of the survival for MM in the recent time periods, presumably related to the availability of new drugs, more widespread use of auto-HCT, and improved supportive care.20,21 The use of allo-HCT in MM has been limited because of reported high TRM rates and the long-term effect on quality of life as a result of chronic GVHD.7,22 The absence of major randomized studies of allo-HCT in newly diagnosed MM (with the notable exception of Bruno et al17 ) has resulted in this modality being used later in the disease course.4-6,16,23,24
Results of this study provide a perspective of the changes in the use and practice of allo-HCT for MM. There is an increasing trend in the age of allo-HC transplant recipients, most probably because of the increasing use of RIC/NMA regimens. This is of particular relevance because the median age at diagnosis of MM is 67 years. Many of the practice trends seen in the current study are not unique to MM and mirror the recently published large single-center experience with allo-HCT for various indications from Seattle.13 The increase in the overall numbers of allo-HCT over time and the increasing number of centers performing the transplantations reflect increasing experience of treating physicians and the recognition of its feasibility in older patients.
No increase was observed in the interval between diagnosis and allo-HCT, despite the increased recent availability of newer nontransplantation treatment options. Increasing appreciation of genetic heterogeneity in myeloma and the poor outcome of patients with high-risk cytogenetic features may have led to these patients receiving transplants earlier.25-28 Our study has the drawback of not having cytogenetic data available for analysis in most of patients because the earlier patient cohorts (1989-1999) were accrued before these tests were routinely performed. Disease characteristics of transplant recipients do not seem to have changed much over the time period. In particular, the lack of change in the IgA population argues against any enrichment for high-risk subgroups such as those with t(4;14) typically associated with an IgA paraprotein.29 The proportion of patients with chemosensitive MM at transplantation did not change between the latter 2 cohorts.
In the recent cohorts, there was a significant increase in the proportion of patients with a prior auto-HCT. This coincides with increasing use of the tandem auto-HCT followed by NMA/RIC allo-HCT and is further supported by the substantial increase in the proportion of allo-HCTs done within 6 months of an auto-HCT. Despite a high proportion of patients on the tandem autologous followed by allo-HCT approach, our data indicate a significant increase in relapse risk of the most recent cohort. This is consistent with the results of the recent BMT CTN 0102 trial as well as a previous report from the European Group for Blood and Marrow Transplantation, both of which showed a high and ongoing risk of relapse after allo-HCT with NMA conditioning.15,30
Several interesting transplant-related practice changes were identified, paralleling overall trends in the allo-HCT field.13,31 The most common regimens used in the recent cohort were low-dose TBI (200 cGy) with or without fludarabine and low-dose melphalan (≤ 150 mg/m2), reflecting the popularity of RIC regimens.32,33 This coincided with decreased use of Cy-TBI and busulfan-Cy regimens, the most common conditioning regimens before 2001.7,34 Another interesting trend is the increasing use of unrelated donors. This is probably a composite effect of older allo-HC transplant recipients (with fewer sibling donors) and increasing probability of finding suitable donors through the NMDP. PBSCs have replaced BM as the source of graft with 88% of transplantations in the latest cohort (compared with 99% marrow grafts in the first cohort).30 There was also decreasing use of methotrexate for GVHD prophylaxis regimens and a increasing use of tacrolimus. These trends are not specific to myeloma and are similar to that seen across other diseases.13
Use of RIC regimens correlated with decreased TRM. Day-100 mortality and the TRM at 1 year have dropped to 17% and 22%, respectively, from 33% in the first cohort for day-100 mortality and 42% in the intermediate cohort for 1-year TRM. Although acute GVHD rates were unchanged during this time frame, the cumulative incidence of chronic GVHD increased. This is most probably explained by the increasing use of PBSC grafts and unrelated donors.
Most importantly, the risk of relapse/disease progression has increased, with the risk nearly doubling for the recent cohort compared with the 1995-2000 cohort. Although this can be explained at least partly by the increasing use of RIC/NMA regimens, the role of patient selection and the more frequent use of antithymocyte globulin may also operate. However, the recent cohort also had a higher proportion of patients receiving the tandem auto-allo approach with a substantial proportion (50%) within 6 months of the auto-HCT. The recent cohort is also more likely to have received modern induction approaches before transplantation. Despite these improvements in induction, the use of tandem auto-allo HCT and lowered TRM, PFS was not improved for the recent cohort of patients with MM. The net result of the decrease in early TRM and the increased relapse rate was an improvement in the 1-year OS rate but similar 3- and 5-year survival. Notably, recently reported results of the BMT-CTN 0102 trial also failed to show any survival benefit for allogeneic approaches, albeit with short follow-up at this time.15 Our study also highlights the high rate of relapse in patients undergoing current allo-HCT regimens for myeloma. The increased risk of relapse seen here and in the recent trials of RIC/NMA transplants, with only a modest decrease in the TRM, does raise the question about the role of NMA transplantations versus myeloablative transplantation for this disease. Given that the most of the allo-SCT for myeloma are done later in the disease course, a myeloablative regimen may provide superior disease control until an immune-mediated graft-versus-tumor effect becomes active.
In conclusion, allo-HCT continues to be used for the treatment of myeloma. Although the recent shift to the use of RIC/NMA regimens has led to a reduction in early TRM, the lack of durable disease control reflected in increased risk of relapse/progression remains a major issue. We recommend allo-HCT for myeloma in the context of clinical trials after the pros and cons of a myeloablative versus RIC/NMA conditioning approach is discussed in detail with the patient. The key area of interest for future allo-HCT trials in MM would be relapse risk reduction by incorporation of newer drugs into different phases of allo-HCT, including conditioning regimen changes and maintenance therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The CIBMTR is supported by the Public Health Service (grant/cooperative agreement U24-CA76518) from the National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); NHLBI and NCI (grant/cooperative agreement 5U01HL069294); Health Resources and Services Administration (HRSA/DHHS; contract HHSH234200637015C); the Office of Naval Research (grants N00014-06-1-0704 and N00014-08-1-0058); and by grants from AABB; Allos Inc; Amgen Inc; anonymous donation to the Medical College of Wisconsin; Astellas Pharma US Inc; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical Inc; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix GmbH; Children's Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Eisai Inc; Genentech Inc; Genzyme Corporation; Histogenetics Inc; HKS Medical Information Systems; Hospira Inc.; Kirin Brewery Co Ltd; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; Millennium Pharmaceuticals Inc; Miller Pharmacal Group; Milliman USA Inc; Miltenyi Biotec Inc; National Marrow Donor Program; Nature Publishing Group; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics Inc; Otsuka America Pharmaceutical Inc; Pall Life Sciences; Pfizer Inc; Schering Corporation; Sigma-Tau Pharmaceuticals; Soligenix Inc; StemCyte Inc; StemSoft Software Inc; Sysmex America Inc; THERAKOS Inc; Vidacare Corporation; ViraCor Laboratories; ViroPharma Inc; and Wellpoint Inc.
The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the US government.
National Institutes of Health
Authorship
Contribution: S.K. and P.N.H. did study design and manuscript preparation; M.-J.Z. and P.L. did the statistical analysis, and all authors participated in interpretation of data and approval of final manuscript.
Conflict-of-interest disclosure: R.P.G. is a paid employee of Celgene Corporation, Summit, NJ. The remaining authors declare no competing financial interests.
Correspondence: Parameswaran N. Hari, Center for International Blood and Marrow Transplantation Research, Medical College of Wisconsin, 9200 W Wisconsin Ave, Ste C5500, Milwaukee, WI 53226; e-mail: phari@mcw.edu.