Abstract
The success of cancer immunotherapy depends on productive tumor cell recognition by killer lymphocytes. γδ T cells are a population of innate-like lymphocytes endowed with strong, MHC-unrestricted cytotoxicity against tumor cells. This notwithstanding, we recently showed that a large proportion of human hematologic tumors is resistant to γδ peripheral blood lymphocytes (PBLs) activated with specific agonists to the highly prevalent Vγ9Vδ2 TCR. Although this probably constitutes an important limitation to current γδ T cell–mediated immunotherapy strategies, we describe here the differentiation of a novel subset of Vδ2− Vδ1+ PBLs expressing natural cytotoxicity receptors (NCRs) that directly mediate killing of leukemia cell lines and chronic lymphocytic leukemia patient neoplastic cells. We show that Vδ1+ T cells can be selectively induced to express NKp30, NKp44 and NKp46, through a process that requires functional phosphatidylinositol 3-kinase (PI-3K)/AKT signaling on stimulation with γc cytokines and TCR agonists. The stable expression of NCRs is associated with high levels of granzyme B and enhanced cytotoxicity against lymphoid leukemia cells. Specific gain-of-function and loss-of-function experiments demonstrated that NKp30 makes the most important contribution to TCR-independent leukemia cell recognition. Thus, NKp30+ Vδ1+ T cells constitute a novel, inducible and specialized killer lymphocyte population with high potential for immunotherapy of human cancer.
Introduction
Tumors develop in hosts endowed with a highly complex immune system that includes various lymphocyte subsets capable of recognizing and destroying transformed cells. It is now widely accepted that, although lymphocytes may constantly patrol tumor formation, cancer cells develop molecular strategies to evade immune surveillance, which are competitively selected under the pressure of the host immune system.1 This dynamic process, termed “cancer immunoediting,” is thought to constitute a major obstacle to cancer immunotherapy.1
Among multiple immune evasion mechanisms, we have recently shown that leukemia and lymphoma primary cells often down-regulate the nonclassical MHC protein, ULBP1, which is critical for recognition of hematologic tumors by γδ T cells expressing the counter-receptor NKG2D.2 γδ T cells are innate-like lymphocytes that account for 1%-10% of peripheral blood lymphocytes (PBL) of healthy people and are capable of targeting a significant fraction of hematologic tumor cell lines tested in the laboratory.3 However, we have demonstrated that many lymphoid leukemia cells are resistant to fully activated Vγ9Vδ2 T cells,2,3 the dominant subset of γδ PBLs. Furthermore, clinical trials involving the in vivo administration of activators of Vγ9Vδ2 T cells have shown limited success, with objective responses restricted to 10%-33% of patients with either hematologic or solid tumors.4-6 Even more modest has been the outcome of trials involving the adoptive transfer of activated and expanded Vδ2+ cells, because no objective responses have been reported.6 In fact, the simple ex vivo expansion of autologous Vδ2+ T cells, whose surveillance the tumor managed to escape in vivo, may be condemned to little therapeutic effect on reinjection into the patient. Therefore, we believe it is critical to invest in strategies that endow γδ T cells with additional recognition machinery to detect tumors that have resisted the natural components present in vivo.
Besides Vγ9Vδ2 T cells, Vδ1+ T cells are also endowed with potent antitumor cytolytic function, particularly as tissue-associated or tumor-infiltrating lymphocytes.7-10 Moreover, Vδ1+ T cells can constitute up to 30% of all γδ PBLs and may thus represent an important alternative population for adoptive cell therapy. However, this possibility remains poorly explored.
In this study we identified and characterized a novel Vδ1+ PBL subset capable of targeting hematologic tumors highly resistant to fully activated Vγ9Vδ2 PBLs. We show that this Vδ1+ population owes its specialized killer function to induced expression of natural cytotoxicity receptors (NCRs), which have been mostly regarded as NK-specific markers. Instead, we show that, although neither Vδ1+ nor Vδ2+ cells express NCRs constitutively, these can be selectively up-regulated in Vδ1+ cells by AKT-dependent signals provided synergistically by γc cytokines (IL-2 or IL-15) and TCR stimulation. We further show that NKp30 and NKp44 are both functional in NCR+ Vδ1+ PBLs, and synergistically contribute to enhanced targeting of lymphocytic leukemia cells, with NKp30 playing the major role in this process. Thus, NKp30+ Vδ1+ PBL constitute a novel promising population for adoptive cell immunotherapy of hematologic malignancies.
Methods
Ethics statement
Research involving clinical samples was conducted according to the principles expressed in the Helsinki Declaration. All procedures were approved by the review board of Instituto Português de Oncologia de Lisboa (Portugal).
Isolation of human peripheral blood γδ T cells
Peripheral blood was collected from anonymous healthy volunteers, diluted in a 1:1 ratio (volume-to-volume) with PBS (Invitrogen Gibco), and centrifuged in Ficol-Paque (Histopaque-1077; Sigma-Aldrich) in a volume ratio of 1:3 (1 part ficol to 3 parts diluted blood) for 30 minutes at 1 500 rpm and 25°C. The interfase containing mononuclear cells was collected and washed (in PBS), and γδ T cells were isolated (to above 95% purity) by magnetic cell sorting via positive selection (with a FITC-labeled anti-TCRγδ antibody) or via negative selection (with a cocktail of Biotin-labeled antibodies; Miltenyi Biotec). When noted, Vδ1+ cells were further purified by magnetic cell sorting via positive selection with a FITC-labeled anti-Vδ1 TCR antibody (Fisher Scientific) and anti-FITC microbeads (Miltenyi Biotec).
Cell culture
Isolated γδ PBLs were cultured at 106 cells/mL at 37°C, 5% CO2 in round-bottom 96 well plates with RPMI 1640 and 2 mM l-glutamine (Invitrogen Gibco) supplemented with 10% FBS (Invitrogen Gibco), 1 mM sodium pyruvate (Invitrogen Gibco), and 50 mg/mL of penicillin and streptomycin (Invitrogen Gibco). The cells were expanded in the presence of 100 U/mL of rhIL-2 (Roche Applied Science), with or without 10 nM of HMB-PP (4-hydroxy-3-methyl-but-2-enyl pyrophosphate; Echelon Biosciences) and 1μg/mL of phytohemagglutinin (PHA; Sigma-Aldrich). Cells were washed and the culture medium was replaced every 5-6 days. To study the induction of NKp30 expression, γδ PBLs were cultured in the presence or absence of 100 U/mL of rhIL-2 (Roche Applied Science), 1μg/mL of soluble anti-CD3 antibody (eBioscience; clone OKT3), and 20ng/mL of rhIL-15 (Biolegend). For TCR blockade, freshly isolated γδ PBL were CFSE-labeled and then incubated for 7 days with anti-TCRγδ (Beckman Coulter; clone IMMU510) diluted 1:20 in complete medium supplemented with 1μg/mL PHA and 100 U/mL rhIL-2. To study the effects of chemical inhibitors of signal transduction, the MEK inhibitor UO126 and the PI-3K inhibitor LY294002 (both from Calbiochem) were added at 10 mM for a 2-hour incubation period and then maintained in culture with 100 U/mL rhIL-2 and 1μg/mL PHA for 7 days.
Flow cytometric cell sorting
For sorting of γδ PBL based on the expression of NKp30 and Vδ1+ TCR, cells from PHA and IL-2–activated cultures were stained with anti-NKp30 (Biolegend; clone P30-15), anti-Vδ1 (Thermo Fisher Scientific; clone TS8.2), and sorted on a FACSAria cell sorter (BD Biosciences).
Leukemia patient samples
B-cell chronic lymphocytic leukemia cells were obtained from the peripheral blood of patients at presentation, after informed consent and institutional review board approval (Instituto Português de Oncologia de Lisboa, Portugal). Samples were enriched by density centrifugation over Ficol-Paque and then washed twice in 10% RPMI 1640.
In vitro tumor-killing assays
All tumor cell lines (details provided in supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) were cultured in complete 10% RPMI 1640, maintained at 105 up to 106 cells/mL by dilution and splitting in a 1:3 ratio every 3-4 days. For cytotoxicity assays, magnetically purified γδ PBL were preactivated for 7-19 days in the presence of IL-2 (100 U/mL) and either 1μg/mL PHA or 10 nM HMB-PP. For receptor blocking, γδ PBLs were incubated for 2 hours with the blocking antibodies anti-NKp30 (clone F252), anti-NKp44 (clone KS38), anti-NKp46 (clone KL247), anti-TCRγδ (Beckman Coulter, clones IMMU510 or B1.1), or anti-Vδ1 TCR (Fisher Scientific, clones TCS1 or TS8.2). The blocking antibodies were maintained in the culture medium during the killing assays. Tumor cell lines or leukemia primary samples were stained with CellTrace Far Red DDAO-SE (1 μM; Molecular Probes, Invitrogen) and each batch of 3 × 104 tumor cells was incubated with 1.5 × 105 to 3 × 105 γδ T cells in RPMI for 3 hours at 37°C and 5% CO2 on a round-bottom plate with 96 wells. Cells were then stained with annexin V–FITC (BD Biosciences) and analyzed by flow cytometry. For the redirected killing assays, PHA and IL-2–activated γδ PBL were incubated for 4 hours with the NCR agonists anti-NKp30 (clone AZ20), anti-Nkp44 (clone Z231) or anti-NKp46 (clone Bab281) during a standard 51Cr release assay.
Flow cytometry analysis
Cells were labeled with the following fluorescent monoclonal antibodies: anti-CD3–PerCP-Cy5.5 (eBioscience; clone OKT3); anti-TCRγδ–FITC (eBioscience; clone B1.1); anti-CD69–PE (BD Pharmingen; clone FN50); anti-NKG2D–PE/Cy7 (Biolegend; clone 1D11); anti-2B4–APC (Biolegend, clone C1.7); anti–DNAM-1–Alexa-Fluor647 (Biolegend; clone DX11); anti-NKp30–APC (Biolegend; clone P30-15); anti-Vδ2 TCR-PE (Biolegend; clone B6); anti-NKp44–APC (Biolegend; clone P44-8); anti-NKp46–AlexaFluor647 (Biolegend; clone 9E2); anti-Vδ1 TCR-FITC (Thermo Fisher Scientific; clone TS8.2); anti-NKp30–PE (Biolegend; clone P30-15); anti–Mouse IgG1κ-APC Isotype Ctrl (Biolegend; clone MOPC-21); anti–Mouse IgG1κ-PE Isotype Ctrl (Biolegend, clone MOPC-21); anti-CD27–APC/Cy7 (Biolegend, clone O323); and anti-CD56–APC (Biolegend, clone HCD56). Cell proliferation was measured by following a standard CFSE staining protocol (CellTrace CFSE Cell Proliferation Kit, Invitrogen; final concentration 0.5 mM), while apoptosis was assessed by annexin V–FITC (BD Pharmingen) staining. Cells were analyzed on a FACSCanto flow cytometer (BD Biosciences).
RNA isolation and cDNA production
Total RNA was extracted using the RNeasy Mini Kit according to the manufacturer's protocol (QIAGEN). Concentration and purity was determined by spectrophotometry and integrity was confirmed using an Agilent 2100 Bioanalyzer with a RNA 6000 Nano Assay (Agilent Technologies). Total RNA was reverse-transcribed into cDNA using random hexamers and Superscript II first strand synthesis reagents (Invitrogen).
Real-time quantitative PCR
Real-time quantitative PCR (qPCR) was performed on ABI Prism 7500 FAST Sequence Detection System using SYBR Green detection system (both from Applied Biosystems). Primers were designed using Primer3 v.0.4.0 online program (http://primer3.sourceforge.net). For each transcript, quantification was done using the calibration curve method. β2-microglobulin (B2M), Glucoronidase β (GUSB) and proteasome subunit β type 6 (PSMB6) were used as housekeeping controls for normalization of gene expression. The following primers were used: B2M, forward CTAT CCAG CGTA CTCC AAAG ATTC, reverse CTTG CTGA AAGA CAAG TCTG AATG; PSMB6, forward GGCG GCTA CCTT ACTA GCTG, reverse AAAC TGCA CGGC CATG ATA; GUSB, forward TGCA GCGG TGTA CTTC TG, reverse CCTT GACA GAGA TCTG GTAA TCA; B7H6, forward TCAC CAAG AGGC ATTC CGAC CT, reverse ACCA CCTC ACAT CGGT ACTC TC; NKP44, forward CCGT CAGA TTCT ATCT GGTG GT, reverse CACA CAGC TCTG GGTC TGG; NKP46, forward AAGA CCCC ACCT TTCC TGA, reverse TGCT GGCT CGCT CTCT AGT; GZMB, forward GGGG GACC CAGA GATT AAAA, reverse CCAT TGTT TCGT CCAT AGGA G. All samples were run in triplicate and repeated 3 times. Analysis of the qPCR results was performed using the ABI SDS v1.1 sequence analysis software (Applied Biosystems).
Statistical analysis.
Differences between subpopulations were assessed using the Student t test and are indicated when significant as *P < .05; **P < .01; and ***P < .001 in the figures.
Results
Enhanced cytotoxicity of γδ PBL cultures activated with pan–T-cell mitogen
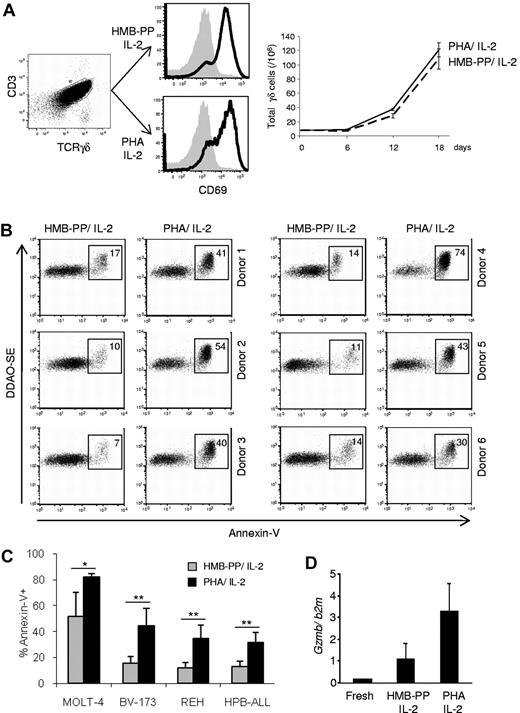
We compared the antitumor killing capacity of γδ PBL cultures (always maintained in the presence of IL-2) activated either with PHA, a plant lectin that acts as a potent T-cell mitogen,11 or the specific Vγ9Vδ2 TCR agonist HMB-PP.12,13 Although both regimens were similarly efficient at activating γδ PBLs, as evaluated by CD69 up-regulation and cell proliferation (Figure 1A), we noted that samples activated with PHA were consistently better killers of hematopoietic tumor cell lines than samples (of the same donor origin) stimulated with HMB-PP (Figure 1B-C). This was valid across all donors tested (Figure 1B, supplemental Table 2, and data not shown) and was associated with higher expression of GZMB (Figure 1D), a key component of the lymphocyte cytolytic machinery. Of note, freshly isolated γδ PBLs, which lack GZMB expression (Figure 1D), displayed very poor antileukemia cytotoxicity (< 10% killing; not shown), as previously reported.13
Enhanced antileukemia cytotoxicity of γδ PBL cultures activated with pan–T-cell mitogen. (A) γδ peripheral blood lymphocytes (γδ PBLs) were MACS-sorted from the peripheral blood of healthy volunteers (left panel), and stimulated with either HMB-PP and IL-2 or PHA and IL-2 for 4 to 19 days. Activation was evaluated by flow cytometry for CD69 up-regulation (middle panels; levels in freshly isolated control cells are shaded), and total cell numbers are shown on the right panel. (B-C) Preactivated (for 14 days, as in panel A) γδ PBLs were coincubated with DDAOse-labeled leukemia cells for 3 hours. Tumor cell lysis was evaluated by annexin-V staining using flow cytometry. (B) Representative results of 6 different donors for the Bv173 leukemia cell line. Percentages refer to annexin-V+ tumor cells. Basal tumor cell apoptosis (in the absence of γδ PBL) was < 5%. (C) Summary of the results of 6 different donors with 4 leukemia target cell lines. Error bars represent SD (n = 6, *P < .05; **P < .01). (D) Real-time PCR quantification of GzmB mRNA levels in freshly isolated, HMB-PP and IL-2–activated and PHA and IL-2–activated γδ PBL. Data in this figure are representative of 2 to 3 independent experiments with similar results.
Enhanced antileukemia cytotoxicity of γδ PBL cultures activated with pan–T-cell mitogen. (A) γδ peripheral blood lymphocytes (γδ PBLs) were MACS-sorted from the peripheral blood of healthy volunteers (left panel), and stimulated with either HMB-PP and IL-2 or PHA and IL-2 for 4 to 19 days. Activation was evaluated by flow cytometry for CD69 up-regulation (middle panels; levels in freshly isolated control cells are shaded), and total cell numbers are shown on the right panel. (B-C) Preactivated (for 14 days, as in panel A) γδ PBLs were coincubated with DDAOse-labeled leukemia cells for 3 hours. Tumor cell lysis was evaluated by annexin-V staining using flow cytometry. (B) Representative results of 6 different donors for the Bv173 leukemia cell line. Percentages refer to annexin-V+ tumor cells. Basal tumor cell apoptosis (in the absence of γδ PBL) was < 5%. (C) Summary of the results of 6 different donors with 4 leukemia target cell lines. Error bars represent SD (n = 6, *P < .05; **P < .01). (D) Real-time PCR quantification of GzmB mRNA levels in freshly isolated, HMB-PP and IL-2–activated and PHA and IL-2–activated γδ PBL. Data in this figure are representative of 2 to 3 independent experiments with similar results.
The superior cytotoxic function of PHA-stimulated γδ PBL cultures was a surprising finding, because we and others have shown that HMB-PP is a very potent activator of the highly dominant Vγ9Vδ2 PBL subset.13 We were particularly interested that, compared with HMB-PP–activated γδ PBL, PHA-stimulated cultures displayed improved cytotoxicity against various resistant leukemia cell lines, such as Bv-173, REH or HPB-ALL (Figure 1B-C), which we had shown to lack expression of the critical NKG2D ligand ULBP1.2,3 Of note, PHA-stimulated γδ T cells did not target normal (healthy) PBMC (supplemental Figure 1). These data demonstrate that the pan–T-cell mitogen PHA is capable of increasing the cytolytic potential of medium-term (1-3 weeks) γδ PBL cultures against leukemia cells, which could be of great value for adoptive cell immunotherapy.
Induction of NCR expression on γδ PBLs activated with pan–T-cell mitogen
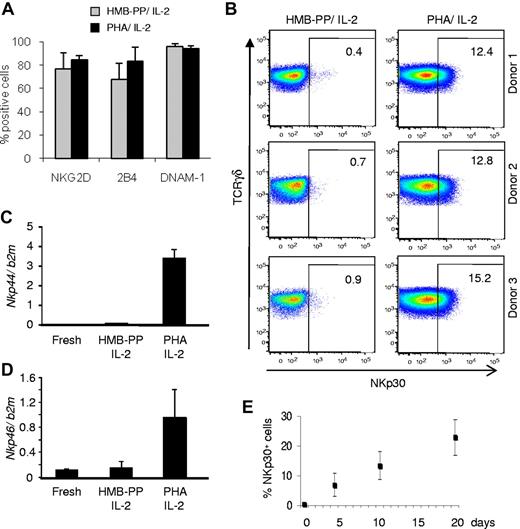
We next investigated the mechanism(s) underlying the enhanced cytotoxicity of PHA-activated γδ PBL cultures. We considered that this could be explained by differential expression of receptors such as NKG2D,2,14,15 DNAM-1,16,17 or 2B4,18 all previously shown to participate in tumor cell recognition by killer lymphocytes. However, none of these candidates was differentially expressed between PHA-activated and HMB-PP–activated γδ PBL cultures (Figure 2A). By contrast, and unexpectedly, the natural cytotoxicity receptor NKp30, an important trigger of NK cell cytotoxicity,19 was specifically found on PHA-stimulated γδ PBLs (Figure 2B; supplemental Figure 2A-B). Furthermore, the other NCR family members, NKp44 and NKp46, were also selectively expressed in these samples (Figure 2C-D; see next paragraph).
Induction of natural cytotoxicity receptor expression in γδ PBLs activated with pan–T-cell mitogen. γδ PBLs were cultured as described in Figure 1 for 4-19 days and analyzed by flow cytometry for surface expression of various NK receptors. (A) Results for NKG2D, 2B4 and DNAM-1 in 10-day cultures activated either with HMB-PP and IL-2 (gray) or PHA and IL-2 (black), derived from 6 independent healthy donors. Error bars represent SD (n = 6; P > .05). (B) Expression of NKp30 in the same cultures of (A). FACS plots correspond to cultures derived from 3 individual donors. Percentages refer to NKp30+ γδ PBLs. Isotype control staining is presented in supplemental Figure 2A. (C-D) Real-time PCR quantification of Nkp44 (C) and Nkp46 (D) mRNA levels in freshly isolated, HMB-PP and IL-2–activated and PHA and IL-2–activated γδ PBL. (E) Evolution of the percentage of NKp30+ cells in the cultures described in (A), analyzed up to day 19. Error bars represent SD (n = 5). Data in this figure are representative of 2 to 4 independent experiments with similar results.
Induction of natural cytotoxicity receptor expression in γδ PBLs activated with pan–T-cell mitogen. γδ PBLs were cultured as described in Figure 1 for 4-19 days and analyzed by flow cytometry for surface expression of various NK receptors. (A) Results for NKG2D, 2B4 and DNAM-1 in 10-day cultures activated either with HMB-PP and IL-2 (gray) or PHA and IL-2 (black), derived from 6 independent healthy donors. Error bars represent SD (n = 6; P > .05). (B) Expression of NKp30 in the same cultures of (A). FACS plots correspond to cultures derived from 3 individual donors. Percentages refer to NKp30+ γδ PBLs. Isotype control staining is presented in supplemental Figure 2A. (C-D) Real-time PCR quantification of Nkp44 (C) and Nkp46 (D) mRNA levels in freshly isolated, HMB-PP and IL-2–activated and PHA and IL-2–activated γδ PBL. (E) Evolution of the percentage of NKp30+ cells in the cultures described in (A), analyzed up to day 19. Error bars represent SD (n = 5). Data in this figure are representative of 2 to 4 independent experiments with similar results.
The proportion of NKp30+ cells increased steadily with culture time (Figure 2E), suggesting an association of NKp30 induction with cell proliferation. Although unlikely because of the very low background in fresh samples (Figure 2E), it was possible that a minute subset constitutively expressing NKp30 could be preferentially expanded in PHA-stimulated γδ PBL cultures. However, experiments with highly (> 99%) FACS-purified NKp30−cells demonstrated that NKp30− cells were able to acquire NKp30 expression as efficiently as unsorted cells on PHA and IL-2 stimulation (supplemental Figure 3). Moreover, under such conditions, NKp30− and NKp30+ cells proliferated to similar extent (not shown), further arguing against preferential expansion of NKp30+ cells under such conditions. These results suggest that NKp30 expression is induced de novo on γδ PBL activation by PHA and IL-2 treatment, which is coupled to cell proliferation.
NCRs are selectively expressed by proliferating Vδ1+ T cells
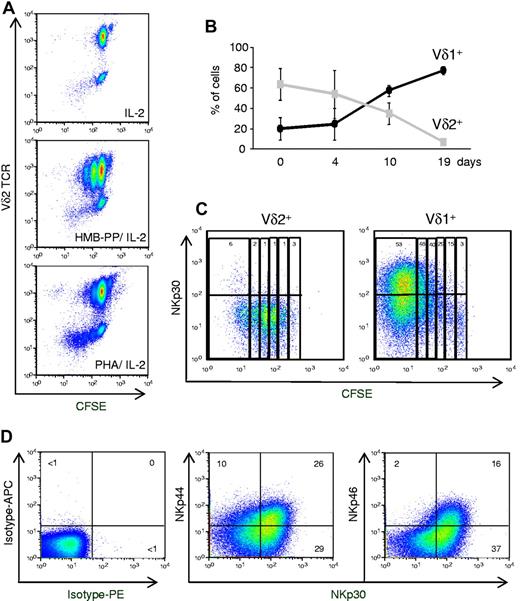
Considering that HMB-PP had been shown to be an optimal agonist of Vγ9Vδ2 cells,12,13 we hypothesized that our findings derived from PHA-mediated activation of a distinct γδ PBL subset. Consistent with this, we observed that, by contrast with HMB-PP, treatment with PHA preferentially expanded Vδ2− cells among γδ PBL (Figure 3A). We verified that this was not because of differences in Vδ2+ cell apoptosis in the 2 experimental conditions (supplemental Figure 2C). The most likely Vδ2− population to expand so markedly (Figure 3A) were Vδ1+ cells, because other subsets are very rare in the peripheral blood of healthy adults.20 When Vδ1 versus Vδ2 TCR usage was assessed, a dramatic Vδ1+ cell enrichment was found in PHA-activated cultures (> 80% of all γδ T cells after 19 days; Figure 3B; supplemental Figure 4). Conversely, and as described,13 HMB-PP–activated cultures were progressively dominated by Vδ2+ cells (Figure 3A; supplemental Figure 4).
Natural cytotoxicity receptors are selectively expressed on proliferating Vδ1+ T cells. (A) γδ PBLs were labeled with CFSE and cultured as described in Figure 1, or in the absence of T cell mitogens (ie, IL-2 alone). Flow cytometry analysis of CFSE dilution and Vδ2 TCR expression after 7 days in culture. (B) Percentage of Vδ1+ or Vδ2+ cells among total γδ PBLs cultured up to 19 days with PHA and IL-2. Error bars represent SD (n = 3). (C) NKp30 expression in PHA and IL-2–activated γδ PBL subsets. Vδ1+ or Vδ2+ cells were FACS-sorted from peripheral blood, labeled with CFSE and cultured with PHA and IL-2 for 7 days. Percentages refer to NKp30+ cells within each cell division (according to CFSE levels and indicated by vertical rectangles). (D) Expression of NKp30, NKp44 and NKp46 in Vδ1+ T cells after 19 days of PHA and IL-2 stimulation. Isotype mAb control stainings are also shown. Data in this figure are representative of 2-3 independent experiments with similar results.
Natural cytotoxicity receptors are selectively expressed on proliferating Vδ1+ T cells. (A) γδ PBLs were labeled with CFSE and cultured as described in Figure 1, or in the absence of T cell mitogens (ie, IL-2 alone). Flow cytometry analysis of CFSE dilution and Vδ2 TCR expression after 7 days in culture. (B) Percentage of Vδ1+ or Vδ2+ cells among total γδ PBLs cultured up to 19 days with PHA and IL-2. Error bars represent SD (n = 3). (C) NKp30 expression in PHA and IL-2–activated γδ PBL subsets. Vδ1+ or Vδ2+ cells were FACS-sorted from peripheral blood, labeled with CFSE and cultured with PHA and IL-2 for 7 days. Percentages refer to NKp30+ cells within each cell division (according to CFSE levels and indicated by vertical rectangles). (D) Expression of NKp30, NKp44 and NKp46 in Vδ1+ T cells after 19 days of PHA and IL-2 stimulation. Isotype mAb control stainings are also shown. Data in this figure are representative of 2-3 independent experiments with similar results.
The induction of NKp30 expression was examined in parallel cultures of isolated Vδ1+ or Vδ2+ cells, which were stimulated with PHA and IL-2. Although neither freshly isolated Vδ1+ nor Vδ2+ cells expressed NKp30 (supplemental Figure 2B), this NCR was strongly induced (on PHA and IL-2 treatment) in Vδ1+ but not Vδ2+ cells (Figure 3C). Moreover, by following CFSE dilution, we demonstrated a striking accumulation of NKp30+ cells with progressive division of Vδ1+ cells (Figure 3C). These data suggest that activation of Vδ1+ cells in PHA and IL-2 cultures induces NKp30 expression concomitantly with cell proliferation.
Whereas high percentages (> 50%) of NKp30+ cells were usually detected after 2 to 3 weeks in culture, NKp44 (∼ 30%) and NKp46 (< 20%) were expressed in lower proportions of Vδ1+ cells (Figure 3D). Furthermore, most of NKp44+ or NKp46+ Vδ1+ cells also expressed NKp30 (Figure 3D). We therefore considered NKp30 as the most informative marker of the inducible NCR+ Vδ1+ subset, and we set out to further characterize its differentiation.
NKp30 induction requires AKT-dependent γc cytokine and TCR signals
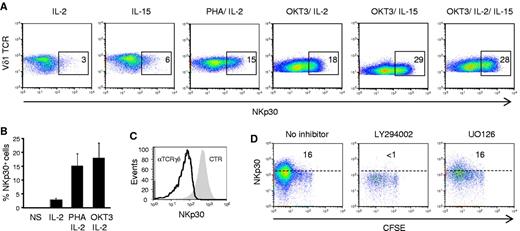
We next dissected the specific signals required for the differentiation of NCR+ Vδ1+ T cells. First, the 2 components of the activation protocol, IL-2 and PHA, were dissociated. IL-2, or its related γc cytokine, IL-15, alone were sufficient to induce some NKp30 expression, but the effect was modest compared with PHA and IL-2 (or PHA and IL-15) combinations (Figure 4A and not shown). On the other hand, PHA alone was not able to keep the cultures viable (data not shown), consistent with the critical role of γc cytokines in the survival of γδ T cells, particularly on activation and proliferation.13,21
AKT-dependent γc cytokine and TCR signals induce NKp30 expression in Vδ1+ T cells. (A-B) Flow cytometry analysis of NKp30 expression on pregated Vδ1+ T cells from γδ PBL cultures after 7 days in the presence of IL-2 or IL-15, alone or in combination with PHA or OKT3 (anti-CD3ϵ mAb). (C) Effect of blocking anti-TCRγδ mAb on NKp30 induction in PHA and IL-2–activated γδ PBLs. The shaded gray area is pregated NKp30+ cells in 7-day control cultures. (D) Effect of chemical inhibitors LY294002 and UO126 on NKp30 induction in PHA and IL-2–activated γδ PBLs, prelabeled with CFSE. Data in this figure are representative of 2 to 3 independent experiments with similar results.
AKT-dependent γc cytokine and TCR signals induce NKp30 expression in Vδ1+ T cells. (A-B) Flow cytometry analysis of NKp30 expression on pregated Vδ1+ T cells from γδ PBL cultures after 7 days in the presence of IL-2 or IL-15, alone or in combination with PHA or OKT3 (anti-CD3ϵ mAb). (C) Effect of blocking anti-TCRγδ mAb on NKp30 induction in PHA and IL-2–activated γδ PBLs. The shaded gray area is pregated NKp30+ cells in 7-day control cultures. (D) Effect of chemical inhibitors LY294002 and UO126 on NKp30 induction in PHA and IL-2–activated γδ PBLs, prelabeled with CFSE. Data in this figure are representative of 2 to 3 independent experiments with similar results.
Although PHA has been a widely used T cell mitogen, it is also a nonphysiologic compound capable of cross-linking a series of surface receptors, including the TCR.22 We hypothesized that the molecular mediator of PHA stimulation could be the Vδ1+ TCR complex. We therefore compared the ability of PHA and the OKT3 mAb, which specifically cross-links CD3ϵ chains of the TCR complex, to induce NKp30 expression (when combined with IL-2 or IL-15) in Vδ1+ T cells. OKT3 was fully capable of mimicking PHA in these assays (Figure 4A-B), thus inducing NKp30 in proliferating Vδ1+ T cells (supplemental Figure 5). Moreover, TCRγδ blockade in PHA and IL-2 cultures prevented NKp30 induction (Figure 4C). These data suggest that PHA treatment provides TCR signals to induce NCR expression on Vδ1+ PBL. Moreover, the differences between cytokine alone or combination treatments with OKT3 (or PHA) highlight a marked synergy between γc cytokine and TCR signals in this process (Figure 4A-B).
To further explore the molecular mechanisms of NCR induction, we used chemical inhibitors of key signal transduction pathways downstream of γc cytokine receptors and/or TCR signaling. Although blocking JAK signaling triggered extensive cell death before any NCR induction (not shown), coincubation with the PI-3K/ AKT inhibitor LY294002 specifically prevented NKp30 induction in proliferating Vδ1+ T cells (Figure 4D). AKT is involved in transducing both γc cytokine and TCR signals,23 including TCRγδ signals.13 By contrast, the MAPK/Erk inhibitor UO126 had no detectable effect on NKp30 induction in proliferating Vδ1+ T cells (Figure 4D). Importantly, the selective effect of LY294002 dissociated NCR induction from cell proliferation, thus demonstrating that Vδ1+ T-cell proliferation is necessary (Figure 3C; supplemental Figure 5) but not sufficient (Figure 4D) to induce NKp30 expression. Collectively, these data demonstrate that AKT-dependent γc cytokine and TCR signals synergize to induce NKp30 expression in Vδ1+ T cells.
Functional NKp30 and NKp44 trigger tumor cell killing by Vδ1+ PBLs
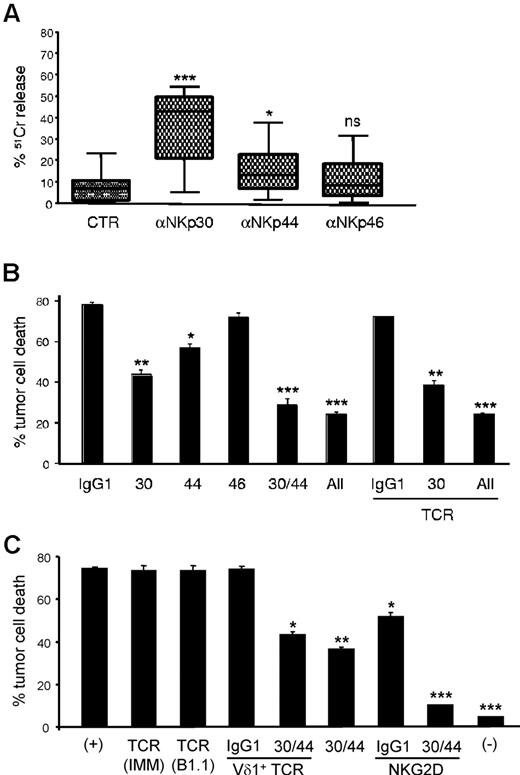
Although the previous data established clear associations between NKp30 expression and increased cytotoxicity of γδ (Vδ1+) PBL cultures, the functional role of NCRs in this system remained to be formally demonstrated. We therefore undertook gain-of function and loss-of-function experiments to evaluate the effect of NCR modulation on Vδ1+ enriched (> 80%; supplemental Figure 3A) PBL cultures, which expressed NCRs at levels similar to those in Figure 3D (not shown). First, using a reverse Ab-dependent cytotoxicity assay, we showed that cross-linking of NKp30 or NKp44, but not NKp46, produced significant increases in lysis of the P815 tumor cell targets (Figure 5A). These data demonstrate that induced NKp30 and NKp44 are functional and mediate tumor cell killing. To assess if they played nonredundant roles in targeting leukemia cells, we performed receptor blockade experiments using NCR-specific mAbs (kindly provided by Dr A. Moretta, University of Genova, Italy). We observed significant reductions in tumor cell lysis on NKp30 and NKp44 blockade (Figure 5B). As expected from the results in Figure 5A, NKp46 blockade did not affect tumor cell killing. Interestingly, a synergistic effect between NKp30 and NKp44 was also clearly observed. Of note, TCRγδ blockade in any setting (alone or in combination with anti-NCR mAbs) was a neutral event during the killing assay (Figure 5B). To further establish the TCR-independence of NCR+ Vδ1+ PBL cytotoxicity, we isolated PHA-activated Vδ1+ cells to very high purity (supplemental Figure 6) and used 3 different well-described anti-TCR blocking antibodies, including one (TCS1) specific for the Vδ1+ TCR. Again, we observed no effect on leukemia cell killing (Figure 5C). By contrast, inhibition of NKG2D had a significant (Figure 5C) and dose-dependent (supplemental Figure 6C) impact on tumor lysis. In fact, when we combined NCR and NKG2D inhibition, NCR+ Vδ1+ PBLs could not kill above background levels (Figure 5C). These data suggest that leukemia cell targeting by NCR+ Vδ1+ PBLs is a TCR-independent event mostly mediated by the synergistic function of NKp30, NKp44 and NKG2D.
NKp30 and NKp44 mediate tumor cell killing by NCR+ γδ PBLs. (A) Functional evaluation of NKp30, NKp44 and NKp46 using specific monoclonal antibodies in a 4-hour 51Cr release redirected killing assay (at 2:1 effector:target ratio) of the FcγR+ P815 target cell line by γδ PBLs activated and expanded with PHA and IL-2. Data are presented as mean and SD of 8 independent experiments performed in triplicate (*P < .05, ***P < .001; ns = not statistically significant). (B) γδ PBLs activated and expanded for 18 days in PHA and IL-2 were incubated (at 5:1 effector:target ratio) for 3 hours with the leukemia cell line MOLT-4 (as in Figure 1). Effect of blocking antibodies to NKp30, NKp44, NKp46 and TCRγδ (IMMU510) on tumor cell killing. (C) Vδ1+ PBLs were MACS-sorted after 20 days in PHA and IL-2 cultures for the assay described in (B), but using blocking antibodies to pan-TCRγδ (IMMU510 and B1.1), Vδ1+ TCR (TCS1), NKG2D or NKp30 and NKp44, or the depicted combinations. (+) refers to control cultures without inhibitory antibodies; (-) refers to tumor cell cultures without Vδ1+ PBLs. Error bars represent SD (n = 3, *P < .05; **P < .01; ***P < .001).
NKp30 and NKp44 mediate tumor cell killing by NCR+ γδ PBLs. (A) Functional evaluation of NKp30, NKp44 and NKp46 using specific monoclonal antibodies in a 4-hour 51Cr release redirected killing assay (at 2:1 effector:target ratio) of the FcγR+ P815 target cell line by γδ PBLs activated and expanded with PHA and IL-2. Data are presented as mean and SD of 8 independent experiments performed in triplicate (*P < .05, ***P < .001; ns = not statistically significant). (B) γδ PBLs activated and expanded for 18 days in PHA and IL-2 were incubated (at 5:1 effector:target ratio) for 3 hours with the leukemia cell line MOLT-4 (as in Figure 1). Effect of blocking antibodies to NKp30, NKp44, NKp46 and TCRγδ (IMMU510) on tumor cell killing. (C) Vδ1+ PBLs were MACS-sorted after 20 days in PHA and IL-2 cultures for the assay described in (B), but using blocking antibodies to pan-TCRγδ (IMMU510 and B1.1), Vδ1+ TCR (TCS1), NKG2D or NKp30 and NKp44, or the depicted combinations. (+) refers to control cultures without inhibitory antibodies; (-) refers to tumor cell cultures without Vδ1+ PBLs. Error bars represent SD (n = 3, *P < .05; **P < .01; ***P < .001).
NKp30+ Vδ1+ PBL are specialized killers that target resistant primary lymphocytic leukemias
To fully characterize the antitumor potential of NCR+ Vδ1+ PBL, we used FACS to sort NKp30+ cells to a high degree of purity (> 99%; Figure 6A) and performed a series of functional assays. As expected (Figure 3D), sorted NKp30+ cells also expressed NKp44 and NKp46 (Figure 6B), and the 3 NCRs were largely stable on the surface of the purified cells when cultured for 2 weeks with IL-2 alone (Figure 6C). These data demonstrate the feasible expansion of a stable NCR+ Vδ1+ T cell subset.
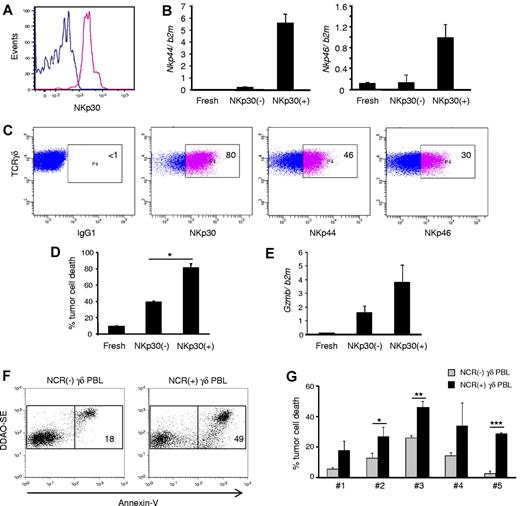
NKp30+ γδ PBLs are a stable subset endowed with enhanced cytotoxicity against chronic lymphocytic leukemia cells. NKp30+ and NKp30(−) γδ PBLs were FACS-sorted from 14-day PHA and IL-2–activated cultures. (A) Reanalysis of NKp30 expression in the purified populations. (B) Real-time PCR quantification of Nkp44 (left) and Nkp46 (right) mRNA levels in NKp30− or NKp30+ γδ T cells, compared with freshly isolated γδ PBLs. Error bars represent SD (n = 3). (C) Sorted NKp30+ γδ PBLs were cultured in the presence of IL-2. Analysis of NKp30, NKp44 and NKp46 expression after 14 days. (D) NKp30− or NKp30+ γδ T cells, or freshly isolated γδ PBLs, were used in killing assays with the leukemia cell line Bv173 (as in Figure 1). Tumor cell death was evaluated by annexin-V staining (n = 3, *P < .05). (E) Real-time PCR quantification of GzmB mRNA levels in freshly isolated, NKp30(−) or NKp30+ γδ T cells. Error bars represent SD (n = 3). (F-G) Representative plots (F) and data summary (G) for 5 primary B-cell chronic lymphocytic leukemia samples that were used in killing assays (as in Figure 1) with γδ PBLs obtained from 6 distinct donors and activated with either HMB-PP and IL-2 or PHA and IL-2. NCR(-) γδ PBL from HMB-PP and IL-2–activated cultures (gray bars) were compared with NCR(+) γδ PBL from PHA and IL-2–activated cultures (black bars). Error bars represent SD (n = 6, *P < .05; **P < .01; ***P < .001).
NKp30+ γδ PBLs are a stable subset endowed with enhanced cytotoxicity against chronic lymphocytic leukemia cells. NKp30+ and NKp30(−) γδ PBLs were FACS-sorted from 14-day PHA and IL-2–activated cultures. (A) Reanalysis of NKp30 expression in the purified populations. (B) Real-time PCR quantification of Nkp44 (left) and Nkp46 (right) mRNA levels in NKp30− or NKp30+ γδ T cells, compared with freshly isolated γδ PBLs. Error bars represent SD (n = 3). (C) Sorted NKp30+ γδ PBLs were cultured in the presence of IL-2. Analysis of NKp30, NKp44 and NKp46 expression after 14 days. (D) NKp30− or NKp30+ γδ T cells, or freshly isolated γδ PBLs, were used in killing assays with the leukemia cell line Bv173 (as in Figure 1). Tumor cell death was evaluated by annexin-V staining (n = 3, *P < .05). (E) Real-time PCR quantification of GzmB mRNA levels in freshly isolated, NKp30(−) or NKp30+ γδ T cells. Error bars represent SD (n = 3). (F-G) Representative plots (F) and data summary (G) for 5 primary B-cell chronic lymphocytic leukemia samples that were used in killing assays (as in Figure 1) with γδ PBLs obtained from 6 distinct donors and activated with either HMB-PP and IL-2 or PHA and IL-2. NCR(-) γδ PBL from HMB-PP and IL-2–activated cultures (gray bars) were compared with NCR(+) γδ PBL from PHA and IL-2–activated cultures (black bars). Error bars represent SD (n = 6, *P < .05; **P < .01; ***P < .001).
When the cytotoxic function of NKp30+ cells was assessed, an increased targeting of the resistant leukemia cell line Bv173 (among others; not shown) was observed (in comparison with NKp30− counterparts; Figure 6D). This correlated with higher expression of granzyme B (Figure 6E). Moreover, NKp30 expression also associated with higher degree of CD56 expression (supplemental Figure 7), which has been previously linked to cytotoxicity of human lymphocytes, including Vδ2+ T cells24 ).
Finally, we performed functional killing assays with primary samples obtained from B-cell chronic lymphocytic leukemia patients. We previously showed that such specimens are considerably resistant to γδ PBL activated and expanded with the specific Vγ9Vδ2 TCR agonist HMB-PP,3 a finding confirmed in this study (Figure 6F-G). Importantly, HMB-PP and IL-2–activated γδ PBLs do not express NCRs (Figure 2B). We therefore compared their antitumor cytolytic activity with that of NKp30+ cells isolated from γδ PBL cultures activated with PHA and IL-2. We observed that NKp30+ γδ PBLs, obtained from 6 different donors, were consistently more efficient at eliminating primary B-CLL cells (Figure 6F-G). These data collectively suggest that highly cytotoxic NKp30+ Vδ1+ PBL are promising new candidates for adoptive cell immunotherapy of hematologic malignancies.
Discussion
Natural cytotoxicity receptors were identified by A. Moretta and colleagues over a decade ago, and were shown to play critical synergistic roles in the antitumor functions of NK cells.19,25-27 In fact, NKp30 and NKp46 are widely considered to be 2 of the most specific NK markers19,25-27 We now show that the combination of cytokine (IL-2 or IL-15) and mitogenic (PHA or OKT3) stimuli induces NCR expression in a sizeable Vδ1+ PBL subset that is endowed with increased cytolytic activity against hematologic tumors. Although PHA is a nonphysiologic T-cell mitogen, we demonstrated that its effect on NCR induction was fully mimicked by crosslinking the TCR-CD3 complex on Vδ1+ PBL. Thus, NCR induction is coupled to TCR-mediated proliferation of Vδ1+ cells, while also requiring γc cytokine signals. This is consistent with previous reports demonstrating that the in vitro acquisition of NK receptors by liver28 or umbilical cord29 T cells depends on IL-15.
Among inducible NCRs, NKp30 is clearly the most important for the antitumor activity of Vδ1+ T cells, based on the proportion of cells that express it (Figure 3D), the higher enhancement in Vδ1+ T-cell cytotoxicity on NKp30 triggering (Figure 5A), and the significant reduction in leukemia cell killing on NKp30 blockade (Figure 5B). This notwithstanding, NKp44 (but not NKp46) is also functional in NCR+ Vδ1+ cells (Figure 5A), and appears to synergize with NKp30 for enhanced tumor targeting (Figure 5B). Of note, NKp30 engagement also augments the production of the key antitumor cytokine, interferon-γ, by NCR+ Vδ1+ cells (supplemental Figure 8).
Both NKp3030 and NKp4431 have been implicated in human NK cell recognition of virus-infected cells. Regarding tumors, antibody-mediated blocking experiments demonstrated important roles for these receptors in myeloma32 and melanoma33 cell targeting. Moreover, lack of NCR expression has been clinically correlated with poor survival in AML patients.34
Interestingly, NKp30 and NKp44 are not encoded in the genome of murine strains (such as C57Bl/6 or Balb/c) widely used for laboratory experimentation.35 On the other hand, the major Vγ9Vδ2 subset of human PBL, and its reactivity toward phosphoantigens, are also primate-specific.6 These observations highlight the special functional characteristics of primate γδ T cells; however, they also preclude the direct in vivo study of Vγ9Vδ2 or NKp30+ Vδ1+ T cells in the mouse.
Another aspect that currently limits our understanding of NCR function in immunity is the poor definition of their physiologic ligands, most notably in the context of tumors. In fact, viral hemagglutinin was until recently the only well-established ligand for NKp4436 and NKp46.37 This notwithstanding, a novel B7 family member, B7-H6, was lately described to bind NKp30.38 B7-H6 was not detected in normal human tissues but was expressed on human tumor cells,38 in line with the recognition of “stressed self” by innate and innate-like lymphocytes.14,39 However, we obtained no evidence for a role of B7-H6 in NCR+ Vδ1+ T-cell recognition of lymphoid leukemias; in fact, many of the NCR+ Vδ1+ T-cell targets expressed lower levels of B7-H6 than healthy control cells (supplemental Figure 9). Brandt et al had also previously noted that only 24 of 119 tumor cell lines tested expressed B7-H6.38 Thus, future lines of research should clarify the repertoire of relevant ligands expressed by tumors susceptible to NKp30-mediated cytotoxicity. Moreover, although we have thus far concentrated on hematologic malignancies, upcoming work will address whether NKp30+ Vδ1+ lymphocytes also possess enhanced cytotoxicity against solid tumors. Of note, Vδ1+ T cells have previously been shown to be cytolytic against melanoma and various carcinomas.10
Vδ1+ T cells are the predominant γδ T-cell subset during the fetal stage and early life,40 when they are already able to respond to viral infection.41 In adults, Vδ1+ T-cell expansions have been associated with CMV infection,42 HIV-1 infection,43 and tumors of either epithelial7-9 or hematopoietic44,45 origin. An attractive prospect for adoptive transfer of activated Vδ1+ T cells is that they may display particularly good capacity for homing to tissues because, contrary to their circulating Vδ2+ counterparts, Vδ1+ cells are preferentially tissue-associated lymphocytes.9 Interestingly, the abundance of Vδ1+ T cells at mucosal surfaces has been attributed to IL-15, which induces chromatin modifications that control TCR gene rearrangement.46
Our key demonstration that NKp30+ Vδ1+ cells are capable of targeting primary lymphoid leukemic cells is particularly relevant when taking into account that Vδ1+ T cells have been previously reported to be inefficient killers of primary leukemia or lymphoma cells,44,45 which has been attributed to their lack of expression of NKG2D ligands.44 Interestingly, a recent study in which γδ PBLs were activated with concanavalin A demonstrated higher killing ability of expanded Vδ1+ cells against B-CLL–derived cell lines.47 It will be important to investigate whether this protocol also induces NKp30 expression on Vδ1+ cells. Thus, NKp30+ Vδ1+ cells may provide a valuable layer of intervention against lymphoid malignancies.
A surprising finding that deserves further investigation is the preferential expansion of Vδ1+ T cells (among γδ PBL) on PHA treatment in vitro (Figure 3B). Because this is not due to selective apoptosis of the dominant Vδ2+ counterparts (supplemental Figure 2), it must derive from a proliferative advantage of Vδ1+ cells when receiving PHA-dependent TCR signals (Figure 3A-B). Provocatively, we have previously observed that Vδ1+ T cells express significantly higher levels of the CD27 receptor (compared with Vδ2+ cells)48 ; CD27 costimulation enhances Bcl2a1 and Cyclin D2 expression and promotes γδ T-cell survival and proliferation.48
This study draws important novel (NCR-mediated) insight into the modus operandis of human γδ T cells, and supports the emerging paradigm of γδ T cells recognizing tumors via innate NK receptors rather than using the somatically rearranged TCRγδ (Figure 5B-C).6 This notwithstanding, the TCR has a major, albeit indirect, contribution to the antitumor function of NKp30+ Vδ1+ cells. We showed that efficient induction of NKp30 expression on Vδ1+ cells depends on TCR stimulation; in its absence, γc cytokines can only effect a very modest up-regulation of NKp30 expression (Figure 4A-B). Thus, TCR signals are upstream of NKp30-mediated tumor cell recognition by NKp30+ Vδ1+ lymphocytes. Interestingly, we have previously showed that, for Vδ2+ cells, TCR signals are essential for cell activation and cytotoxic differentiation, “upstream” of tumor cell recognition via NKG2D.2 We therefore propose a “2-step” model for human γδ T cells, in which they differentiate and are activated like prototypic T cells (ie, using the TCR), but rely essentially on NK receptors (such as NKG2D, NKp30 or DNAM-1) for tumor cell recognition. This is consistent with the critical role described by the Hayday and Girardi groups for NKG2D ligands in tumor surveillance by mouse γδ T cells,49,50 and fits the general concept of NK receptors being the key molecular recognition determinants of “oncogenic stress.”39
From a clinical perspective, this study describes a protocol to induce NKp30 ex vivo, which should make it very feasible to expand and inject large numbers of cells into patients. Importantly, we were able to efficiently expand NKp30+ Vδ1+ cells from B-CLL patients (supplemental Figure 10). On reinfusion, the activation status of the cells could potentially be maintained via administration of low doses of IL-2, which appears to be sufficient to sustain NKp30 expression. Thus, after this first report describing the differentiation of NKp30+ Vδ1+ lymphocytes, future work should evaluate their potential for adoptive cell immunotherapy of human cancer.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Telma Lança, Haakan Norell, and Margareta Correia for suggestions; Alessandro Moretta and João T. Barata for materials; and the Serviço de Hematologia of Instituto Português de Oncologia de Lisboa for provision of leukemia samples.
This work was supported by the Young Investigator Program of the European Molecular Biology Organization and Fundação Calouste Gulbenkian SDH Oncologia 2008/99293 (B.S.-S.); and by the Italian Ministry of Health (Ricerca Finalizzata, ICH-2007-643769), the European Union (Marie Curie International Reintegration Grant, 204188), and Istituto Clinico Humanitas (Intramural Research Program 20090917; D.M.). B.S.-S. is also funded by the European Research Council (StG260352), and D.V.C. is a PhD fellow of Fundação para a Ciência e Tecnologia (SFRH/BD/37898/2007).
Authorship
Contribution: D.V.C., M.F., and K.H. performed the experiments and analyzed the data; M.G.S. provided vital materials and technical help; D.M. and B.S.-S. designed the study and analyzed the data; and B.S.-S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M.F. is the Department of Experimental and Applied Medicine, University of Brescia, Brescia, Italy.
Correspondence: Bruno Silva-Santos, Unidade de Imunologia Molecular, Instituto de Medicina Molecular, Avenida Prof Egas Moniz, 1649-028 Lisboa, Portugal; e-mail: bssantos@fm.ul.pt.
References
Author notes
D.M. and B.S.-S. contributed equally to this study.