Abstract
The opioid system (opioid peptides and receptors) regulates a variety of neurophysiologic functions, including pain control. Here we show novel roles of the κ opioid system in vascular development. Previously, we revealed that cAMP/protein kinase A (PKA) signaling enhanced differentiation of vascular progenitors expressing VEGF receptor-2 (fetal liver kinase 1; Flk1) into endothelial cells (ECs) through dual up-regulation of Flk1 and Neuropilin1 (NRP1), which form a selective and sensitive VEGF164 receptor. Kappa opioid receptor (KOR), an inhibitory G protein–coupled receptor, was highly expressed in embryonic stem cell–derived Flk1+ vascular progenitors. The addition of KOR agonists to Flk1+ vascular progenitors inhibited EC differentiation and 3-dimensional vascular formation. Activation of KOR decreased expression of Flk1 and NRP1 in vascular progenitors. The inhibitory effects of KOR were reversed by 8-bromoadenosine-3′,5′-cAMP or a PKA agonist, N6-benzoyl-cAMP, indicating that KOR inhibits cAMP/PKA signaling. Furthermore, KOR-null or dynorphin (an endogenous KOR agonist)–null mice showed a significant increase in overall vascular formation and ectopic vascular invasion into somites at embryonic day −10.5. ECs in these null mice showed significant increase in Flk1 and NRP1, along with reciprocal decrease in plexinD1, which regulates vascular pathfinding. The opioid system is, thus, a new regulator of vascular development that simultaneously modifies 2 distinct vascular properties, EC differentiation and vascular pathfinding.
Introduction
Opioids are defined by their ability to bind to and influence opioid receptors on cell membranes. Three opioid receptors, μ, δ, and κ (MOR, DOR, and KOR), are inhibitory G (Gi) protein-coupled receptors through which endogenous opioids (endorphins, enkephalins, and dynorphins) regulate physiologic functions, such as pain regulation, emotional tone, and reward circuitry.1 These opioid receptors are the principle physiologic target for most clinically important opioid analgesics, such as morphine. Opioid systems are mainly present in neural tissue and could be involved in neurogenesis during brain development.2,3 We previously showed that DOR, but not MOR or KOR, plays a crucial role in neurogenesis and neuroprotection in neural stem cells obtained from embryonic C3H mouse forebrain.4 In recent studies that used embryonic stem (ES) cells, MOR and KOR were shown to promote neural progenitor differentiation and regulate cell specification from neural progenitors.5,6 Moreover, KOR is highly expressed in human neural precursor cells and functions during neurogenesis in the fetal brain.7 These results indicate that the opioid systems are involved in neurogenesis from neural stem and progenitor cells.
Although endogenous opioids were first characterized in the brain, these opioid systems are found both in neural (brain and spinal cord) and extraneural tissues (ganglia, gut, spleen, stomach, lung, pancreas, liver, heart, blood, and blood vessels). Opioids and opioid receptors were reported to exist in blood vessels in the later stage rat embryo (embryonic day [E]−16) through adult.8,9 The addition of opioid peptides inhibited angiogenesis in a chick chorioallantoic membrane model10 and DNA synthesis in rat vascular walls.8 In the adult, the endogenous opioid system has been shown to be active in hemodynamic and cardiovascular responses, such as hemorrhagic shock, sepsis, and trauma.11 The selective κ opioid receptor agonist U-50,488H has beneficial effects on vascular injury after spinal cord trauma by improving vascular permeability and edema.12 These findings suggest that the opioid system plays an important role in vascular functions though its physiologic roles and molecular mechanisms remain largely unknown.
VEGF/VEGF receptor-2 (fetal liver kinase 1; Flk1) signaling is a key regulator of vascular development during embryogenesis. A VEGF coreceptor, Neuropilin1 (NRP1), is largely co-expressed with Flk1 in vascular progenitors and forms a specific and sensitive receptor for VEGF164, an isoform of VEGF.13,14 The Flk1-VEGF164-NRP1 complex potently enhances Flk1 signaling in vascular development.13 NRP1 is also expressed in particular classes of developing neurons and functions as a receptor for class 3 semaphorins by forming a heterodimer with plexins.15-17 PlexinD1 and semaphorin regulate not only brain development but also angiogenesis and vascular pathfinding.17-20 Recently, various findings suggest that blood vessels and nerves share similar molecular machinery for their differentiation and patterning, including the Semaphorin-plexin/NRP, Ephrin-Eph, Slit-Robo, and Netrin-Unc5 networks.21
We previously demonstrated that Flk1+ cells derived from ES cells serve as vascular progenitors and can constructively reproduce early vascular organization processes, including the differentiation of both endothelial cells (ECs) and mural cells (MCs; vascular smooth muscle cells and pericytes) and vascular structure formation.22 We recently reported that the activation of cAMP/protein kinase A (PKA) signaling markedly increased the sensitivity of Flk1+ vascular progenitors to VEGF through dual up-regulation of Flk1 and NRP1 and played a pivotal role in EC differentiation.23 Opioid signaling inhibits adenylyl cyclase through Gi protein and subsequently decreases cAMP production and inactivates PKA,1 implying involvement of opioids in EC differentiation and vascular formation.
In the current study, we investigated the roles of the opioid system in vascular development through the combined use of our ES-cell differentiation system and 2 knockout (KO) mice models for components of the κ opioid system. KOR, but not MOR or DOR, was highly expressed in vascular progenitors and negatively regulated EC differentiation and in vitro vascular formation via inhibition of cAMP/PKA signaling. KOR-null or dynorphin (endogenous KOR agonist)–null mice showed a significant increase in vascular formation in the early embryos, reflecting the inhibitory effects on EC differentiation and vascular formation of KOR in vitro. Moreover, ectopic vascular invasion into somites of E−10.5 embryos accompanied by decreased plexinD1 expression in ECs was observed in both KO mice, suggesting the involvement of KOR in vascular pathfinding. The opioid system, thus, can be a dual modifier of EC differentiation and vascular pathfinding.
Methods
Antibodies
Monoclonal antibodies for murine Flk1 (AVAS12) and murine vascular endothelial (VE)–cadherin (VECD1, for FACS) were as described previously.24 Monoclonal antibodies for murine CD31 (1:500), VE-cadherin (for immunostaining, 1:200) were purchased from BD Pharmingen. Monoclonal antibodies for murine α-smooth muscle actin (SMA; 1:1000) were from Sigma-Aldrich. Polyclonal antibodies for murine VEGF and rat NRP1 were from R&D Systems. Polyclonal antibodies for murine μ, δ, and κ opioid receptor were from Neuromics.
Cell culture and differentiation
ES cell lines EStTA-ROSA were maintained as described elsewhere.23,25 Differentiation was induced in these ES cell lines by the use of differentiation medium (DM; α-MEM; Gibco) supplemented with 10% FCS (Japan Bioserum) and 5 × 10−5 M 2-mercaptoethanol (Gibco) as described previously.22,23,25 In brief, undifferentiated ES cells were cultured on gelatin-coated dishes without leukemia inhibitory factor at a cell density of 0.75-1 × 103 cells/cm2 for 96-108 hours. Cultured cells were harvested and subjected to purification by MACS. Purified Flk1+ cells were then plated onto gelatin-coated dishes (Becton Dickinson) at a density of 0.75-1 × 104 cells/cm2 in DM. After 3 days of Flk1+ cell differentiation (Flk-d3), induced ECs were examined by immunohistochemistry and flow cytometric analyses.
Various reagents were occasionally added to the Flk1+ cell culture, including human VEGF165 (R&D Systems); 8-bromoadenosine-3′,5′-cyclic monophosphate sodium salt (8br-cAMP; Nacalai Tesque); N6-benzoyl-cAMP (6-Bnz-cAMP; PKA agonist; Biolog); [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO; Sigma-Aldrich); [(+)-4-[(aR)-a-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide] (SNC80); (−)-trans-(1S,2S)-U-50 488 hydrochloride (U50,488H); and 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[N-methyl-trans-3-(3-furyl) acrylamido]morphinan hydrochloride (TRK820; Toray).
3D culture
Three-dimensional (3D) culture was performed as described previously.22,23 In brief, Flk1+ cells (4 × 105 cells/mL) were incubated in DM with VEGF on uncoated Petri dishes for 16 hours to induce aggregation. Aggregates were resuspended in 2 × DM and mixed with an isovolume of collagen I-A gel (3 mg/mL; Nitta Gelatin Inc). We plated 250-300 μL of this mixture onto a lucent insert disk, Cell disk (Sumitomo Bakelite), in 24-well dishes. After 30 minutes at 37°C to allow polymerization, we added 500 μL of DM. To monitor vascular formation, collagen-embedded Flk1+ cell aggregates were cultured in a temperature- and gas-controlled chamber (37°C, 5% CO2) for up to 5 days.
FACS analysis and cell purification
After Flk1+ cells were induced, they were harvested and stained with allophycocyanin (APC)–conjugated AVAS12 antibody.22 Flk1+ cells were sorted by auto MACS (Miltenyi Biotec) with the use of anti-APC MicroBeads (Miltenyi Biotec) and recultured for EC differentiation. Cultured cells were harvested at Flk-d3 and stained with a combination of PE-conjugated anti-CD31 MoAb (Mec13.3; BD Pharmingen), APC-conjugated anti–VE-cadherin MoAb, and/or APC-conjugated AVAS12 antibody and then subjected to analysis with the use of FACS Aria (Becton Dickinson). CD31-positive and CD31-negative cells, respectively, were purified with FACS Aria.
Immunohistochemistry
Immunostaining for cultured cells was performed as described previously.22,23,26 In brief, 4% paraformaldehyde-fixed cells were blocked by 1% skim milk (BD Biosciences) and incubated overnight with primary antibodies at 4°C. For immunofluorescent staining, anti–mouse, anti–rat, anti–rabbit, or anti–goat IgG antibodies conjugated with Alexa488 or Alexa546 (Invitrogen) were used for secondary antibodies. Nuclei were visualized with DAPI (Invitrogen). Stained cells were photographed with inverted fluorescent microscopy (Eclipse TE2000-U; Nikon) and digital camera system (AxioCam HRc) with the use of AxioVision Version 4.7.1 software (Carl Zeiss).
Immunostaining for 3D vascular structures in type I collagen gel and embryos was performed after the whole-mount immunostaining procedure.22,23 In brief, gels were fixed with 4% paraformaldehyde, blocked by 1% skim milk/0.1% Triton X/PBS solution, and incubated with anti-CD31 (BD Pharmingen) at 4°C. For immunofluorescent staining, Alexa Fluor 488– or 546–conjugated anti–rat antibody was used as a secondary antibody. Stained cells were photographed by inverted fluorescent microscopy (BZ-9000; Keyence). The results of quantification of 3D vascular formation were expressed as the CD31+ cell area and CD31+ vascular sprouting length using BZ-H1C software (Keyence). Only extension of CD31+ cells from the aggregates but not CD31+ cells in aggregate area was assessed to evaluate vascular formation. The results of quantification of embryo vascular formation were expressed as the CD31+ cell area with the use of BZ-H1C software (Keyence).
RNA isolation, RT-PCR, and quantitative RT-PCR
Total RNA was isolated from cells in undifferentiated ES cells, Flk1-positive cells, Flk-d1 cells, Flk-d3 cells, and CD31-positive and CD31-negative cells induced from ES cells and purified ECs derived from mice by the use of RNeasy (QIAGEN), according to the manufacturer's instructions. Reverse transcription was performed with the SuperScript III first-strand synthesis system (Invitrogen). RT-PCR was carried out as described by the use of the indicated primers (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Quantitative RT-PCR was performed by the use of Power SYBR Green PCR Master Mix (Applied Biosystems) and a StepOnePlus system (Applied Biosystems). The amount of target RNA was determined from the appropriate standard curve and normalized relative to the amount of GAPDH mRNA. Primer sequences are shown in supplemental Table 2.
Mice
All animal experiments were performed in accordance with the guidelines for Animal Experiments of Kyoto University and Hoshi University, which conform to the Guide for the Care and Use of Laboratory Animals in Japan. Prodynorphin (PDYN) gene-KO mice (The Jackson Laboratory),27,28 KOR gene-KO mice (The Jackson Laboratory),29 or C57BL/6J mice (CLEA Japan Inc) were used. Mice were allowed to mate naturally at night. E0.5 was considered to be noon on the day a vaginal plug was observed. Embryos at E8.75, E10.5, and E12.5 were subjected to immunostaining. Embryos at E10.5 were also subjected to EC (CD31+/VE-cadherin+/CD45−) analysis and sorting with FACS.
Statistical analysis
At least 3 independent experiments were performed. Statistical analysis of the data was performed with ANOVA. P < .05 was considered significant. Values are reported as means ± SEM.
Results
KOR plays an inhibitory role in EC differentiation and vascular formation
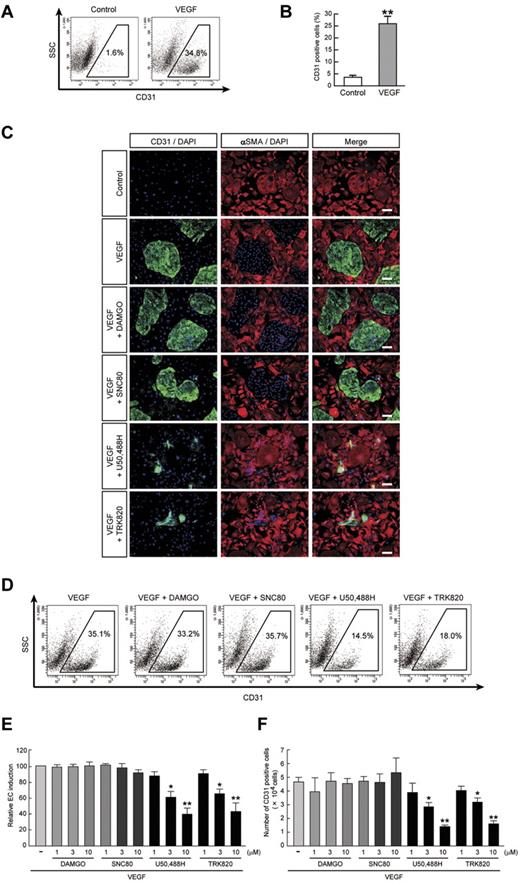
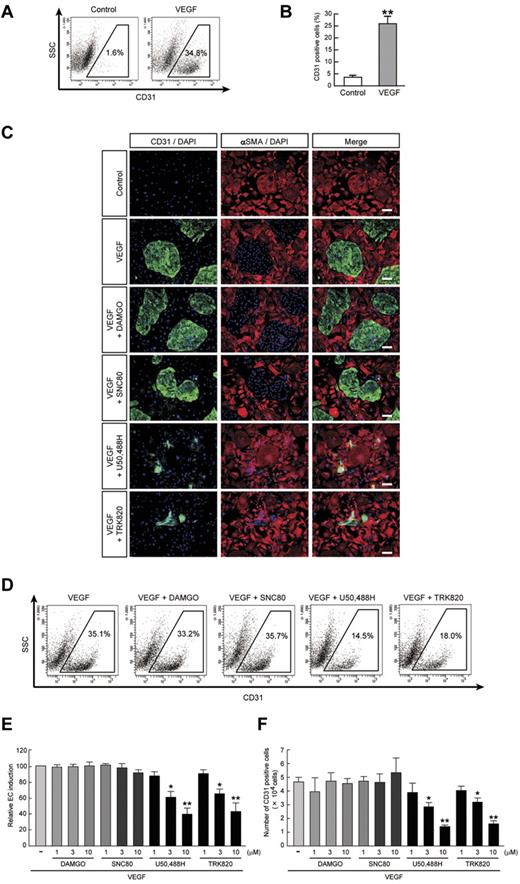
We previously reported that 2 vascular cell types, ECs (positive for CD31/VE-cadherin/endothelial nitric oxide synthase/Claudin5) and MCs (positive for SMA/Calponin/SM22α), were selectively induced when purified ES cell–derived Flk1+ cells were cultured with VEGF and serum.22,23 Although Flk1+ cell culture with serum alone induced predominantly MC differentiation, the addition of VEGF significantly induced EC differentiation compared with serum alone conditions (percentage of CD31+ ECs; 3.5% ± 0.9% in control vs 25.4% ± 3.0% in VEGF, n = 7, **P < .01; Figure 1A-B).
Inhibitory effects of KOR agonists U50,488H and TRK820 on EC induction from Flk1+ cells. Flk1+ cells after 3 days of culture (Flk-d3). (A) Flow cytometry. X-axis: CD31; y-axis: side scatter (SSC). Percentages of CD31+ ECs among total Flk1+ cell–derived cells are indicated. (B) Quantitative evaluation of the effect of VEGF on CD31+ EC induction from Flk1+ cells by FACS. Percentages of CD31+ cell population among total Flk1+ cell–derived cells. Control (n = 7) and VEGF (50 ng/mL; n = 7) treatments are shown (**P < .01 vs control). (C) Double fluorescent staining for CD31 and αSMA at Flk-d3. Left, CD31 (pan-ECs, green) and DAPI (blue); middle, αSMA (MCs, red) and DAPI (blue); and right, merged. Flk1+ cells were stimulated with VEGF (50 ng/mL), DAMGO (10μM), SNC80 (10μM), U50,488H (10μM), or TRK820 (10μM) as indicated. Scale bars, 200 μm. (D) Flow cytometry. X-axis: CD31; y-axis: SSC. Percentages of CD31+ ECs among total Flk1+ cell–derived cells are indicated. (E) Percentages of CD31+ cell population among total Flk1+ cell–derived cells. Treatments with VEGF alone (50 ng/mL; n = 7), and VEGF plus DAMGO (1, 3, 10μM; n = 4), SNC80 (1, 3, 10μM), U50,488H (1, 3, 10μM; n = 4), or TRK820 (1, 3, 10μM; n = 4) are shown (**P < .01, *P < .05 vs VEGF). (F) CD31+ cell number that appeared from 1.5 × 105 of Flk1+ cells. (**P < .01, *P < .05 vs VEGF).
Inhibitory effects of KOR agonists U50,488H and TRK820 on EC induction from Flk1+ cells. Flk1+ cells after 3 days of culture (Flk-d3). (A) Flow cytometry. X-axis: CD31; y-axis: side scatter (SSC). Percentages of CD31+ ECs among total Flk1+ cell–derived cells are indicated. (B) Quantitative evaluation of the effect of VEGF on CD31+ EC induction from Flk1+ cells by FACS. Percentages of CD31+ cell population among total Flk1+ cell–derived cells. Control (n = 7) and VEGF (50 ng/mL; n = 7) treatments are shown (**P < .01 vs control). (C) Double fluorescent staining for CD31 and αSMA at Flk-d3. Left, CD31 (pan-ECs, green) and DAPI (blue); middle, αSMA (MCs, red) and DAPI (blue); and right, merged. Flk1+ cells were stimulated with VEGF (50 ng/mL), DAMGO (10μM), SNC80 (10μM), U50,488H (10μM), or TRK820 (10μM) as indicated. Scale bars, 200 μm. (D) Flow cytometry. X-axis: CD31; y-axis: SSC. Percentages of CD31+ ECs among total Flk1+ cell–derived cells are indicated. (E) Percentages of CD31+ cell population among total Flk1+ cell–derived cells. Treatments with VEGF alone (50 ng/mL; n = 7), and VEGF plus DAMGO (1, 3, 10μM; n = 4), SNC80 (1, 3, 10μM), U50,488H (1, 3, 10μM; n = 4), or TRK820 (1, 3, 10μM; n = 4) are shown (**P < .01, *P < .05 vs VEGF). (F) CD31+ cell number that appeared from 1.5 × 105 of Flk1+ cells. (**P < .01, *P < .05 vs VEGF).
To investigate whether the opioid system is involved in EC differentiation, we examined the effects of a MOR agonist, DAMGO, a DOR agonist, SNC80, and KOR agonists U50,488H and TRK820 on EC differentiation in our system. The addition of U50,488H or TRK820 to Flk1+ vascular progenitors inhibited CD31+ EC induction from Flk1+ cells (Figure 1C). These effects were inhibited by treatment with nor-binaltorphimine, a selective KOR antagonist (supplemental Figure 1A). Moreover, knockdown of KOR expression by transfection of siRNA for KOR abolished the effects of U50,488H and TRK820, indicating that the effects of KOR agonists are mediated by KOR (supplemental Figure 1B-C). In contrast, DAMGO or SNC80 did not inhibit EC induction.
Quantitative evaluation with the use of FACS analysis demonstrated that VEGF with 10μM U50,488H or TRK820 treatment reduced the EC population by approximately 40% compared with VEGF alone (CD31+ cell population relative to VEGF alone; 39.7% ± 8.0% [10μM U50,488H treatment, n = 8], 43.3% ± 10.5% [10μM TRK820 treatment, n = 6], *P < .05, **P < .01; Figure 1D-E). The total number of ECs that appeared from the same number of Flk1+ cells was decreased to approximately 30% by treatment with U50,488H or TRK820 (CD31+ ECs; 4.6 ± 0.4 × 104 cells [VEGF alone, n = 8] vs 1.4 ± 0.1 × 104 cells [VEGF with U50,488H, 10μM, n = 8] or 1.6 ± 0.2 × 104 cells [VEGF with TRK820, 10μM, n = 6], *P < .05, **P < .01; Figure 1F). In contrast, the total number of MCs was not changed by treatment with U50,488H or TRK820 (data not shown). The addition of KOR agonists showed no effect on either the proliferation of ECs and MCs when evaluated with the BrdU incorporation assay or on apoptosis when evaluated with the TUNEL assay at Flk-d1 and Flk-d3 (supplemental Figures 2-3). These results indicated that KOR signaling specifically inhibits the induction of ECs from Flk1+ progenitor cells.
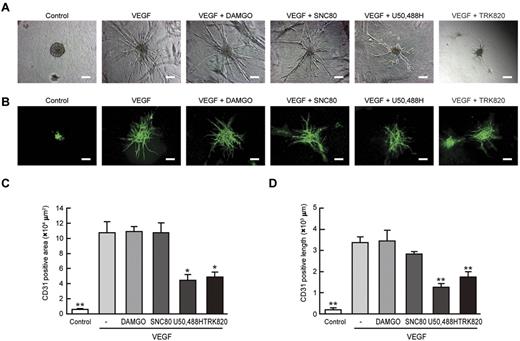
We further examined vascular formation from Flk1+ cells in 3D culture. When aggregates of Flk1+ cells were cultured in type I collagen gel, no sprouting of vessels was observed in culture with serum alone. In contrast, treatment with VEGF induced the formation of vascular-like structures with CD31+ ECs (Figure 2 A-B).22,23 The addition of U50,488H or TRK820 to Flk1+ cell aggregates significantly inhibited vascular formation. The area of CD31+ cells and the length of CD31+ vascular sprouting were reduced to approximately 50% of those under treatment with VEGF alone (Figure 2C-D). In contrast, DAMGO or SNC80 did not inhibit 3D vascular formation.
Inhibitory effects of KOR agonists U50,488H and TRK820 on 3D vascular formation from Flk1+ cells. Three-dimensional culture of Flk1+ cell aggregates in type I collagen gel. (A) Phase contrast images after 5 days of culture. Flk1+ cells were stimulated with control, VEGF (50 ng/mL), DAMGO (10μM), SNC80 (10μM), U50,488H (10μM), or TRK820 (10μM) as indicated. Scale bars, 200 μm. (B) In-gel immunostaining for CD31 (green). Scale bars, 200 μm. (C) Quantitative analysis of CD31+ area in 3D vascular formation (except for the aggregate area). Control (n = 3), VEGF alone (50 ng/mL; n = 3), and VEGF plus DAMGO (10μM; n = 3), SNC80 (10μM; n = 3), U50,488H (10μM; n = 3), or TRK820 (10μM; n = 3) are shown (**P < .01, *P < .05 vs control). (D) Quantitative analysis of length in CD31+ sprouting vessels (excluding the aggregate area; **P < .01 vs VEGF).
Inhibitory effects of KOR agonists U50,488H and TRK820 on 3D vascular formation from Flk1+ cells. Three-dimensional culture of Flk1+ cell aggregates in type I collagen gel. (A) Phase contrast images after 5 days of culture. Flk1+ cells were stimulated with control, VEGF (50 ng/mL), DAMGO (10μM), SNC80 (10μM), U50,488H (10μM), or TRK820 (10μM) as indicated. Scale bars, 200 μm. (B) In-gel immunostaining for CD31 (green). Scale bars, 200 μm. (C) Quantitative analysis of CD31+ area in 3D vascular formation (except for the aggregate area). Control (n = 3), VEGF alone (50 ng/mL; n = 3), and VEGF plus DAMGO (10μM; n = 3), SNC80 (10μM; n = 3), U50,488H (10μM; n = 3), or TRK820 (10μM; n = 3) are shown (**P < .01, *P < .05 vs control). (D) Quantitative analysis of length in CD31+ sprouting vessels (excluding the aggregate area; **P < .01 vs VEGF).
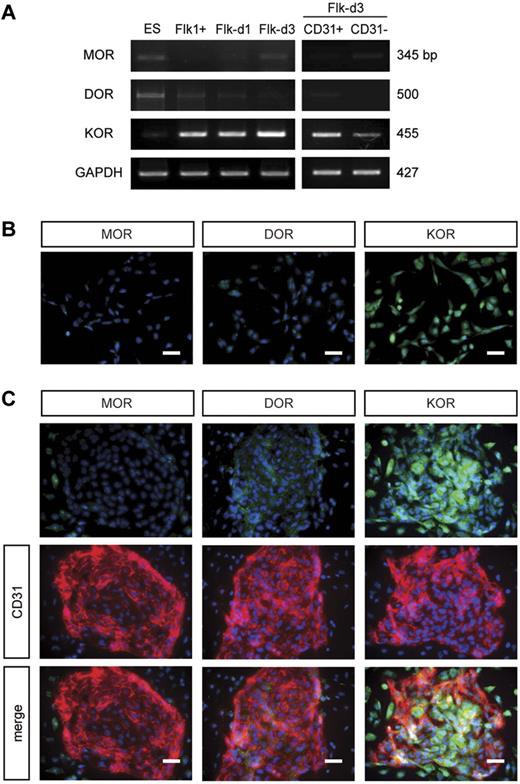
Next, we confirmed opioid receptor expression during EC differentiation. Although 3 opioid receptor mRNAs were expressed at low levels in undifferentiated ES cells, KOR, but not MOR or DOR, was highly expressed in Flk1+ vascular progenitors and during EC differentiation (Flk-d1-d3). A lower KOR expression was observed in CD31− cells at Flk-d3 (Figure 3A). Reflecting mRNA expressions, KOR protein was detected in both CD31+ and CD31− cells, whereas MOR and DOR were not at Flk-d3 (supplemental Figure 4). We further confirmed the time course of opioid receptor expression in the early stage of Flk1+ cell culture by immunostaining. Cells at Flk-d1 highly expressed KOR but not MOR or DOR (Figure 3B). Although KOR was highly expressed in ECs and MCs at Flk-d3, MOR and DOR were expressed at a low level in ECs and MCs, respectively (Figure 3C). These results suggest that the predominant expression of KOR in Flk1+ vascular progenitors should be involved in EC differentiation and vascular formation.
KOR was highly expressed in Flk1+ vascular progenitors. (A) RT-PCR showing mRNA expression of MOR, DOR, and KOR in ES cells, Flk1+ cells, cells after 1 or 3 days of Flk1+ cell culture (Flk-d1 or Flk-d3), CD31-positive cells (ECs) and CD31-negative cells (MCs) at Flk-d3. (B) Fluorescent staining for MOR, DOR, and KOR at Flk-d1. Nuclei are stained with DAPI (blue). Left, MOR; middle, DOR; right, KOR. Scale bars, 100 μm. (C) Double fluorescent staining for MOR, DOR, and KOR with CD31 (red) at Flk-d3. Nuclei are stained with DAPI (blue). Top, opioid (green) receptors (green); middle, CD31 (red); bottom, merged. Scale bars, 100 μm.
KOR was highly expressed in Flk1+ vascular progenitors. (A) RT-PCR showing mRNA expression of MOR, DOR, and KOR in ES cells, Flk1+ cells, cells after 1 or 3 days of Flk1+ cell culture (Flk-d1 or Flk-d3), CD31-positive cells (ECs) and CD31-negative cells (MCs) at Flk-d3. (B) Fluorescent staining for MOR, DOR, and KOR at Flk-d1. Nuclei are stained with DAPI (blue). Left, MOR; middle, DOR; right, KOR. Scale bars, 100 μm. (C) Double fluorescent staining for MOR, DOR, and KOR with CD31 (red) at Flk-d3. Nuclei are stained with DAPI (blue). Top, opioid (green) receptors (green); middle, CD31 (red); bottom, merged. Scale bars, 100 μm.
Activation of cAMP/PKA signaling rescues the effects of KOR on EC induction from Flk1+ vascular progenitors
Opioid receptors transduce signals through Gi protein to inhibit adenylyl cyclase and subsequently decrease cAMP production and inactivate PKA. We measured intracellular cAMP concentration on KOR activation (supplemental Figure 5). Whereas the addition of U50,488H or TRK820 to Flk1+ cells decreased intracellular cAMP concentration, additional treatment with a KOR antagonist, BNI, reversed the inhibitory effects (supplemental Figure 5A). Furthermore, the addition of U50,488H or TRK820 to purified ECs (CD31+ cells) or MCs (CD31− cells) showed that KOR agonists significantly decreased cAMP concentration in ECs but not in MCs (supplemental Figure 5B-C), indicating that KOR negatively regulates cAMP concentration by directly affecting ECs.
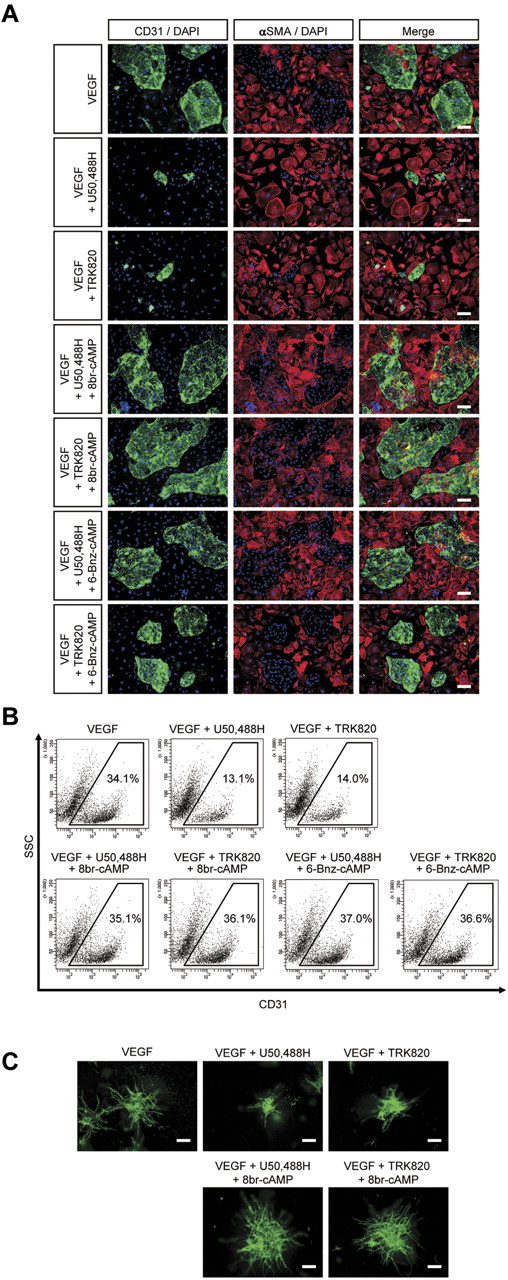
We investigated whether cAMP/PKA signaling activation can rescue the inhibitory effect of KOR on EC induction and vascular formation. The appearance of ECs as examined by immunostaining and FACS analysis demonstrated that the inhibitory effects of U50,488H and TRK820 on CD31+ EC induction were completely rescued by treatment with a cAMP analog, 8br-cAMP, or a PKA agonist, 6-Bnz-cAMP (Figure 4A-B). Similarly, vascular formation from Flk1+ cells in 3D culture inhibited by U50,488H or TRK820 was completely rescued by 8br-cAMP (Figure 4C). These results indicate that the inhibitory effect of KOR on vascular development is mediated by the suppression of cAMP/PKA signaling through Gi activation in vascular progenitors.
Activation of cAMP/PKA signaling rescues the inhibitory effects of KOR on EC induction from Flk1+ vascular progenitors. (A) Double fluorescent staining for CD31 and αSMA at Flk-d3. Left, CD31 (pan-ECs, green) and DAPI (blue); middle, αSMA (MCs, red) and DAPI (blue); and right, merged. Flk1+ cells stimulated with VEGF (50 ng/mL), U50,488H (10μM), TRK820 (10μM), 8br-cAMP (0.5mM), and 6-Bnz-cAMP (0.1mM) as indicated. Scale bars, 200 μm. (B) Flow cytometry. X-axis: CD31; y-axis: SSC. Percentages of CD31+ ECs among total Flk1+ cell–derived cells are indicated. (C) Three-dimensional culture of Flk1+ cell aggregates in type I collagen gel. In-gel immunostaining for CD31 (green). Scale bars, 200 μm.
Activation of cAMP/PKA signaling rescues the inhibitory effects of KOR on EC induction from Flk1+ vascular progenitors. (A) Double fluorescent staining for CD31 and αSMA at Flk-d3. Left, CD31 (pan-ECs, green) and DAPI (blue); middle, αSMA (MCs, red) and DAPI (blue); and right, merged. Flk1+ cells stimulated with VEGF (50 ng/mL), U50,488H (10μM), TRK820 (10μM), 8br-cAMP (0.5mM), and 6-Bnz-cAMP (0.1mM) as indicated. Scale bars, 200 μm. (B) Flow cytometry. X-axis: CD31; y-axis: SSC. Percentages of CD31+ ECs among total Flk1+ cell–derived cells are indicated. (C) Three-dimensional culture of Flk1+ cell aggregates in type I collagen gel. In-gel immunostaining for CD31 (green). Scale bars, 200 μm.
The VEGF-A receptors Flk1 and NRP1 are decreased by KOR activation in Flk1+ vascular progenitors
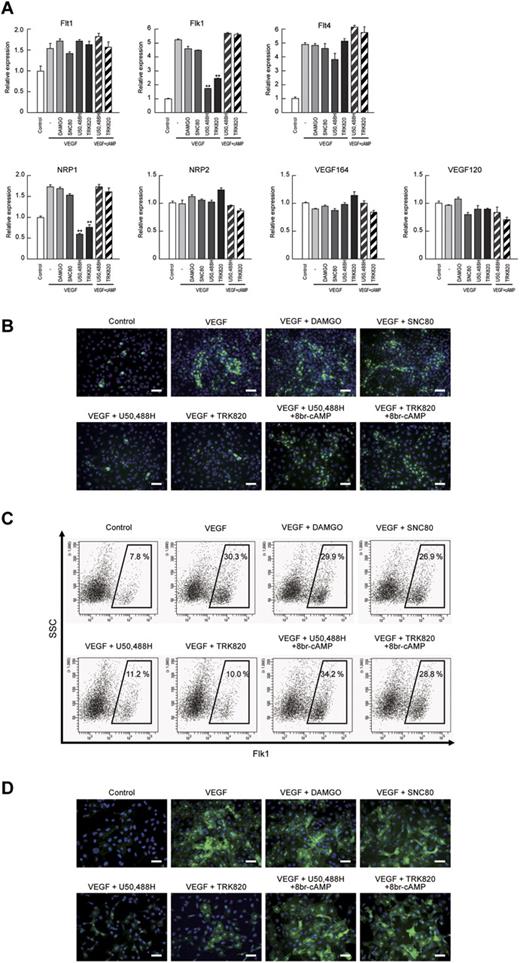
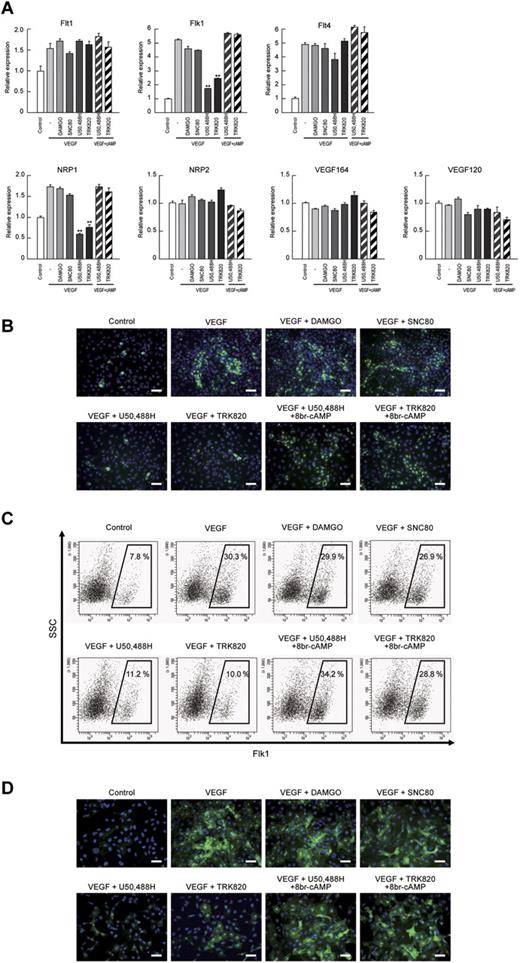
We previously reported that cAMP/PKA signaling increased both Flk1 and NRP1 expression in vascular progenitors and markedly enhanced the “sensitivity” of the progenitors to VEGF164 by inducing Flk1-VEGF164-NRP1 complex formation.23 Therefore, we examined VEGF ligand and VEGF receptor expression after the activation of opioid receptors. Both Flk1 and NRP1 mRNAs were specifically down-regulated by the addition of U50,488H or TRK820, but not by DAMGO or SNC80, in the early stage of Flk1+ cell culture (Flk-d1; Figure 5A) and early ECs (Flk-d3; supplemental Figure 6). The inhibitory effects of KOR on Flk1 and NRP1 expression were reversed by treatment with 8br-cAMP, indicating that KOR inhibits Flk1 and NRP1 expression through cAMP signaling in EC differentiation (Figure 5A). We further examined the expression of Flk1 and NRP1 by immunostaining and FACS analysis at Flk-d1. Almost all Flk1+ cells were still negative for the EC markers CD31 and VE-cadherin at Flk-d1, suggesting that these cells have not yet committed to EC at this stage.23 U50,488H and TRK820, but not DAMGO or SNC80, suppressed Flk1 expression as detected by immunostaining and Flk1+ cell appearance in FACS analysis (Figure 5B-C). U50,488H or TRK820 similarly suppressed NRP1 expression (Figure 5D). Treatment with 8br-cAMP rescued the inhibitory effects of KOR on Flk1 and NRP1 expression (Figure 5B-D). These results indicate that KOR signaling regulates vascular formation through Flk1 and NRP1 expression in vascular progenitors.
VEGF receptors Flk1 and NRP1 were decreased by KOR activation in Flk1+ vascular progenitors. (A) Quantitative RT-PCR showing mRNA expression at Flk-d1. Control mRNA expression was set as 1.0. Flk1+ cells were stimulated with control, VEGF (50 ng/mL), DAMGO (10μM), SNC80 (10μM), U50,488H (10μM), TRK820 (10μM), 8br-cAMP (0.5mM), and 6-Bnz-cAMP (0.1mM) as indicated (**P < .01 vs VEGF). (B) Fluorescent staining for Flk1 (green) at Flk-d1. Nuclei are stained with DAPI (blue). Scale bars, 200 μm. (C) Flow cytometry at Flk-d1. X-axis: Flk1; y-axis: SSC. Percentages of Flk1+ cells among vascular progenitor-derived cells are indicated. (D) Fluorescent staining for NRP1 (green) at Flk-d1. Nuclei are stained with DAPI (blue). Scale bars, 200 μm.
VEGF receptors Flk1 and NRP1 were decreased by KOR activation in Flk1+ vascular progenitors. (A) Quantitative RT-PCR showing mRNA expression at Flk-d1. Control mRNA expression was set as 1.0. Flk1+ cells were stimulated with control, VEGF (50 ng/mL), DAMGO (10μM), SNC80 (10μM), U50,488H (10μM), TRK820 (10μM), 8br-cAMP (0.5mM), and 6-Bnz-cAMP (0.1mM) as indicated (**P < .01 vs VEGF). (B) Fluorescent staining for Flk1 (green) at Flk-d1. Nuclei are stained with DAPI (blue). Scale bars, 200 μm. (C) Flow cytometry at Flk-d1. X-axis: Flk1; y-axis: SSC. Percentages of Flk1+ cells among vascular progenitor-derived cells are indicated. (D) Fluorescent staining for NRP1 (green) at Flk-d1. Nuclei are stained with DAPI (blue). Scale bars, 200 μm.
KOR or dynorphin KO mice show increased vascular formation in the early embryos
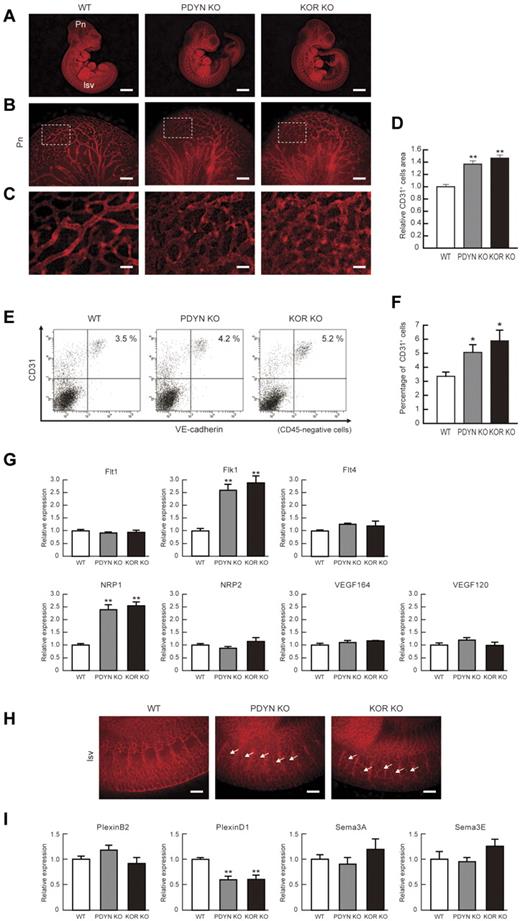
Finally, we confirmed the in vivo effects of KOR on vascular formation using KO animal models for KOR and precursor PDYN, which encodes for dynorphin, an endogenous KOR agonist. Both KOR and PDYN KO mice are viable, with reported phenotypic effects on neural function such as enhancement of pain perception.1,27-29 Vascular phenotype, however, has not been investigated in either of these KO mice. Both KOR and PDYN KO mice showed a greater density of CD31+ vessels in the periphery of E−10.5 embryonic brains (Figure 6B-C). Quantitative evaluation of CD31+ area demonstrated a significant increase in blood vessels in embryos from both KO mice (CD31+ cell area relative to that in wild-type [WT] mice; 1.37 ± 0.05 in KOR KO mice, 1.47 ± 0.05 in PDYN KO mice, n = 3 [independent pregnant mice], **P < .01; Figure 6D).
KOR or Dynorphin KO mice show increased vascular formation in brain and intersomitic vessels. (A) Representative results of WT, PDYN KO, and KOR KO mouse embryo at E10.5. Whole-mount CD31 (red) staining. Left, WT mice. Pn, perineural vascular plexus; Isv, intersomitic vessels. Middle, PDYN KO mice. Right, KOR KO mice. Scale bars, 2 mm. (B) High-magnification views of CD31-stained Pn region. Scale bars, 200 μm. (C) Greater magnification views corresponding to boxed regions in panel B. Scale bars, 40 μm. (D) Quantitative evaluation of CD31+ area in Pn. CD31 staining of WT mice was set as 1.0. (n = 3, **P < .01 vs WT). (E) Flow cytometry. X-axis: VE-cadherin; y-axis: CD31. Percentages of CD31+/VE-cadherin+/CD45− ECs in the embryo are indicated. (F) Quantitative evaluation of CD31+/VE−cadherin+/CD45− ECs in the embryo. (n = 3, *P < .05 vs WT). (G) Quantitative RT-PCR showing mRNA expression in purified CD31+/VE-cadherin+/CD45− ECs in the embryo. WT mRNA expression was set as 1.0. (n = 3, **P < .01 vs WT). (H) High-magnification views of CD31-stained Isv region in E10.5 embryo. Ectopic invasion of Isv into somite was observed in both KO mice. Scale bars, 100 μm. (I) Quantitative RT-PCR showing mRNA expression in purified CD31+/VE−cadherin+/CD45− ECs in the embryo. WT mRNA expression was set as 1.0. (n = 3, **P < .01 vs WT).
KOR or Dynorphin KO mice show increased vascular formation in brain and intersomitic vessels. (A) Representative results of WT, PDYN KO, and KOR KO mouse embryo at E10.5. Whole-mount CD31 (red) staining. Left, WT mice. Pn, perineural vascular plexus; Isv, intersomitic vessels. Middle, PDYN KO mice. Right, KOR KO mice. Scale bars, 2 mm. (B) High-magnification views of CD31-stained Pn region. Scale bars, 200 μm. (C) Greater magnification views corresponding to boxed regions in panel B. Scale bars, 40 μm. (D) Quantitative evaluation of CD31+ area in Pn. CD31 staining of WT mice was set as 1.0. (n = 3, **P < .01 vs WT). (E) Flow cytometry. X-axis: VE-cadherin; y-axis: CD31. Percentages of CD31+/VE-cadherin+/CD45− ECs in the embryo are indicated. (F) Quantitative evaluation of CD31+/VE−cadherin+/CD45− ECs in the embryo. (n = 3, *P < .05 vs WT). (G) Quantitative RT-PCR showing mRNA expression in purified CD31+/VE-cadherin+/CD45− ECs in the embryo. WT mRNA expression was set as 1.0. (n = 3, **P < .01 vs WT). (H) High-magnification views of CD31-stained Isv region in E10.5 embryo. Ectopic invasion of Isv into somite was observed in both KO mice. Scale bars, 100 μm. (I) Quantitative RT-PCR showing mRNA expression in purified CD31+/VE−cadherin+/CD45− ECs in the embryo. WT mRNA expression was set as 1.0. (n = 3, **P < .01 vs WT).
To further evaluate overall vascular formation in the embryo, we dissociated whole E10.5 embryos and quantitatively assessed total EC appearance by FACS. The total percentages of ECs from whole embryo preparations were significantly increased in both KO mice compared with WT (percentage of CD31+/VE-cadherin+/CD45− ECs; 3.33% ± 0.33% [WT], 5.07% ± 0.52% [PDYN KO], 5.87% ± 0.77% [KOR KO], n = 3 [independent pregnant mice], *P < .05; Figure 6E-F). These results indicate that vascular formation was enhanced in both KO mice at the whole embryo level. We confirmed the expression of VEGF ligands and receptors in ECs purified from E−10.5 embryos. Purified ECs from both KOR and PDYN KO mice showed a specific increase in Flk1 and NRP1 (Figure 6G).
We next examined embryos in the earlier stage (E-8.75) or the later stage (E-12.5) of KOR and PDYN KO mice. E-8.75 embryos of PDYN or KOR KO mice also showed a greater density of CD31+ vessels in the periphery brains relative to WT mice (CD31+ cell area relative to that in WT mice; 1.73 ± 0.1 in KOR KO mice, 1.62 ± 0.12 in PDYN KO mice, n = 3 [independent pregnant mice], **P < .01; supplemental Figure 7A-C). However, E−12.5 embryos of PDYN or KOR KO mice showed almost no vascular phenotypes in brain and intersomites (supplemental Figure 7D-G). These results suggested that KOR signaling could be mainly involved in early stages of vascular development.
Previously we demonstrated that cAMP signaling induces arterial ECs from vascular progenitors through activation of Notch and β-catenin signaling.25,26 We confirmed arterial-venous development in KOR and PDYN KO mice. Expressions of various arterial EC marker mRNAs were not different in purified ECs from WT and 2 KO mice. No apparent morphologic change was observed in large arteries and veins in both KO mice (supplemental Figure 8), suggesting that KOR is not involved in arterial-venous specification.
To our surprise, in both KOR and PDYN KO mice, intersomitic vessels (ISVs) invaded and extended throughout each somite at E10.5 (Figure 6H). Because the ectopic invasion of ISV suggests an abnormality in attractive-repulsive signaling such as delta-like (Dll)/Notch and semaphorin/plexin,17-20,30 we checked Dll/Notch and semaphorin/plexin expression in purified ECs from embryos. The expression of Notch1, Notch4, Dll1, and Dll4 did not change in ECs purified from embryos (supplemental Figure 9). Interestingly, plexinD1 expression in purified ECs was significantly down-regulated in both KOR and PDYN KO mice compared with WT mice, although the expressions of PlexinB2, Semaphorine3A, and Semaphorin3E were unchanged (Figure 6I). We further confirmed plexinD1 expression in ES cell–derived ECs. PlexinD1 expression was also significantly down-regulated in ECs induced by VEGF plus 8br-cAMP compared with those in ECs induced by VEGF alone (supplemental Figure 10). These results suggest that the KOR system is also involved in EC pathfinding through the regulation of plexinD1 expression in the early stages of EC differentiation.
Discussion
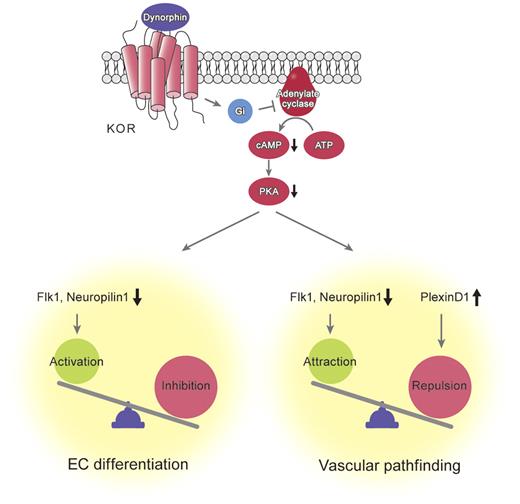
In this study, we demonstrated a novel mechanism for the regulation of EC differentiation and vascular formation through the opioid system. KOR activation suppressed EC differentiation by decreasing the VEGF-A receptors Flk1 and NRP1 through inhibition of cAMP/PKA signaling. KOR and PDYN KO mice mutually showed that endogenous κ opioid signaling plays critical roles in vascular formation and pathfinding; to our knowledge, this is the first report of a molecule involved in both EC differentiation and vascular pathfinding (Figure 7). Novel roles of the opioid system as a negative regulator of angiogenic properties in the early EC linage should provide unique insight into vascular development.
Molecular mechanisms of inhibition of vascular formation and pathfinding by KOR signaling activation. KOR signaling induced by dynorphin activates cAMP/PKA pathway. This pathway reciprocally regulates expression of VEGF receptors, Flk1 and Neuropilin1, and plexinD1 in vascular progenitors. Expression balance of these receptors controls vascular formation and pathfinding during vascular development.
Molecular mechanisms of inhibition of vascular formation and pathfinding by KOR signaling activation. KOR signaling induced by dynorphin activates cAMP/PKA pathway. This pathway reciprocally regulates expression of VEGF receptors, Flk1 and Neuropilin1, and plexinD1 in vascular progenitors. Expression balance of these receptors controls vascular formation and pathfinding during vascular development.
Endogenous angiogenesis inhibitors, such as thrombospondin-1, endostatin, tumstatin, chondromodulin-1, and vasohibin, are naturally present in blood flow and possess antiangiogenic activity and may counterbalance angiogenesis stimulators such as VEGF, basic fibroblast growth factor, among others.31,32 Thus, a physiologic balance in angiogenesis is maintained by angiogenic and antiangiogenic factors. We demonstrated that the κ opioid acts as a novel antiangiogenic factor by changing the characteristic of vascular progenitors and ECs through inhibition of cAMP/PKA signaling. We recently found that cAMP/PKA signaling in vascular progenitors increased the VEGF-A receptors, Flk1 and NRP1, which enhance EC differentiation and vascular formation through increased sensitivity of vascular progenitors to VEGF.23 The κ opioid system is the first identified endogenous inhibitory machinery to cAMP/PKA function in EC and vascular progenitors.
ISV formation is strictly controlled by attractive-repulsive signaling. Zebrafish plexinD1 mutants display spatially unrestricted migration of angioblasts from the dorsal aorta. The timing of ISV migration in plexinD1 mutants is earlier than that of WT zebrafish.18,20 Mouse embryos deficient for plexinD1 have a similar phenotype, also exhibiting excessive ISV branching.17,19 These findings indicate that endothelial plexinD1 mainly contribute to proper ISV formation. In both KOR and PDYN KO mice, the ectopic invasion of ISV to somites, similar to that in plexinD1-deficient mice, was observed, even though the expression of plexinD1 in ECs was reduced to only 50% of that in the control. Interestingly, a recent study suggested that the balance of NRP1 and plexinD1 expression is critical for the function of plexinD1.33 That is, semaphorin3E (sema3E), which binds to both plexinD1 and NRP1, exerts different responses in plexinD1-expressing neuronal populations depending on the presence or absence of the NRP1. In corticofugal and striatonigral neurons that do not contain NRP1, sema3E/plexinD1 signaling produces a “repulsive” response of growing axons. In contrast, in subiculomamillary neurons that express NRP1, sema3E/plexinD1 signaling causes an “attractive” response.33 Both KOR KO and PDYN KO mice showed up-regulated NRP1 (approximately 2-fold change) and reciprocally down-regulated plexinD1 (approximately 50% change) in purified embryo ECs, resulting in an approximately 4-fold increase in the NRP1/plexinD1 ratio. Such a change in the balance between NRP1 and plexinD1 may enhance the ISV phenotype in KOR and PDYN KO mice.
Although the similar phenotypes in both KOR and PDYN KO mice indicate the significance of κ opioid system in vascular development, these phenotypes were transient. To investigate compensatory mechanisms for κ opioid signaling in ECs, we examined expression of 3 opioid receptors in purified ECs from E10.5 and E12.5 embryos. Although KOR was constantly expressed at E10.5 and E12.5 in WT mice, MOR and DOR were expressed at very low levels at E10.5 but increased at E12.5 (supplemental Figure 11A). In both PDYN and KOR KO mice, MOR and DOR expression in ECs at E12.5 also was greater than that at E10.5 (supplemental Figure 11A-B). Such increase in MOR and DOR in the later stage ECs of the embryo should be involved in the compensation of vascular phenotypes at E12.5.
Opioids have been readily applied in clinical use. Morphine, a classic MOR agonist, is currently one of the most effective drugs available clinically for the management of moderate-to-severe pain, including pain associated with cancer. Recently, Koodie et al34 reported that the administration of morphine suppressed tumor angiogenesis by inhibiting VEGF transcription and secretion in hypoxic condition. We confirmed that KOR, but not MOR, was highly expressed in various ECs such as HUVECs (data not shown), suggesting that KOR agonists could directly act on tumor ECs to suppress VEGF receptor expression, similar to the effects observed in embryonic ECs. If so, a combination therapy including an MOR agonist, morphine, and a KOR agonist (such as TRK820, a clinically approved drug in Japan for uremic pruritus) may prove useful for cancer therapy through the suppression of tumor angiogenesis by dual inhibition of VEGF ligands and receptors, extending the therapeutic benefits beyond pain relief. KOR activation reduced VEGF receptor expressions in our results, but Singleton et al35,36 reported that methylnaltrexone, a peripheral MOR antagonist, inhibits VEGF-induced angiogenesis through inhibition of intracellular VEGF receptor signaling. A more precise and careful evaluation would be required for understanding such complicated regulation and relationship between opioids and VEGF signaling and the final outcome in clinical use of opioids. Thus, our novel findings in this study extend not only our understanding of developmental biology but also possible therapeutic applications of opioids to vascular manipulation strategies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Knut Woltjen and Dr Meiko Takahashi, from Kyoto University, for their critical reading of the manuscript.
This study was supported by grants from the Ministry of Education, Science, Sports, and Culture of Japan; the Ministry of Health, Labor, and Welfare of Japan; the Project for Realization of Regenerative Medicine; and the Japan Society for the Promotion of Science.
Authorship
Contribution: K.Y. performed all experiments and wrote the manuscript; S.F. helped with experiments and regent preparation; S.F., S.K., and Michiko Narita helped with ES-cell differentiation, and cell sorting; Michiko Narita, N.K., and S.I. helped with the preparation of KO mice; H.N. supplied TRK820 regents; and T.S., Mimoru Narita, and J.K.Y supervised all experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Minoru Narita, PhD, Department of Toxicology, Hoshi University School of Pharmacy and Pharmaceutical Sciences 2-4-41 Ebara, Shinagawa-ku, Tokyo 142-8501, Japan; e-mail: narita@hoshi.ac.jp; and Jun K. Yamashita, MD, PhD, Laboratory of Stem Cell Differentiation, Stem Cell Research Center, Institute for Frontier Medical Sciences, Kyoto University, 53 Shogoin Kawahara-cho, Sakyo-ku, Kyoto 606-8507, Japan; e-mail: juny@frontier.kyoto-u.ac.jp.