Abstract
In mammals, stromal cell–derived factor-1 (SDF-1) promotes hematopoietic cell mobilization and migration. Although the zebrafish, Danio rerio, is an emerging model for studying hematopoietic cell transplantation (HCT), the role of SDF-1 in the adult zebrafish has yet to be determined. We sought to characterize sdf-1 expression and function in the adult zebrafish in the context of HCT. In situ hybridization of adult zebrafish organs shows sdf-1 expression in kidney tubules, gills, and skin. Radiation up-regulates sdf-1 expression in kidney to nearly 4-fold after 40 Gy. Assays indicate that zebrafish hematopoietic cells migrate toward sdf-1, with a migration ratio approaching 1.5 in vitro. A sdf-1a:DsRed2 transgenic zebrafish allows in vivo detection of sdf-1a expression in the adult zebrafish. Matings with transgenic reporters localized sdf-1a expression to the putative hematopoietic cell niche in proximal and distal renal tubules and collecting ducts. Importantly, transplant of hematopoietic cells into myelosuppressed recipients indicated migration of hematopoietic cells to sdf-1a–expressing sites in the kidney and skin. We conclude that sdf-1 expression and function in the adult zebrafish have important similarities to mammals, and this sdf-1 transgenic vertebrate will be useful in characterizing the hematopoietic cell niche and its interactions with hematopoietic cells.
Introduction
The zebrafish, Danio rerio, is emerging as a useful model organism for the study of hematopoietic cell transplantation (HCT).1,2 Its fecundity makes high experimental sample numbers logistically feasible, it is amenable to high-throughput chemical screens for hematopoietic effects of drugs and small molecules,3 and its optical transparency offers excellent opportunities for direct visualization of cell migration events and in vivo fluorescence detection of donor-derived hematopoietic reconstitution. The true extent to which the zebrafish will prove useful for experimental HCT studies, however, will ultimately depend on the degree to which the mechanisms that result in HCT success in mammals are conserved in the zebrafish.
In mammals, the chemokine stromal cell–derived factor-1 (SDF-1; CXCL12) and its cognate receptor CXCR4 have been strongly implicated in the homing and reconstitution that that occurs during HCT.4-6 SDF-1 is expressed in many organs throughout the body, including the spleen, thymus, skin, heart, lung, kidney, and bone marrow.7,8 The widespread anatomic distribution of SDF-1 expression has been shown to play roles in diverse migration and retention events of a wide array of hematopoietic cells, including migration of mast cell precursors in the skin,9 retention of myeloid cells in extramedullary locations,10 migration of megakaryocytes in the bone marrow after radiation,11 migration of hematopoietic stem cells (HSCs) to regions of injury,12 and HSC migration to bone marrow both during homeostasis as well as during HCT.13,14
In the bone marrow cavity, cells expressing high levels of SDF-1 help to form the HSC niche and play important roles in hematopoietic stem and progenitor cell (HSPC) support.5 In addition to high expression of SDF-1, concomitant expression of several cell adhesion molecules and cell surface receptors in the Notch signaling pathway probably contribute to conditions necessary for survival and maintenance of HSCs.15,16 The exact identity of high SDF-1–secreting cells is unknown, but these functions have been ascribed to stromal cells, mesenchymal stem cells, perivascular cells, and osteoblasts, all of which have been reported to compose or contribute to an HSC niche.17,18 Although previous work indicates conservation of some aspects of SDF-1–driven neutrophil hematopoiesis and migration in the zebrafish,19 the full extent to which the hematopoietic roles of the zebrafish sdf-1/Cxcr4 axis functionally mirrors that of mammals remains to be seen.
The hematopoietic organ of the adult zebrafish is the kidney, which contains renal tubules interspersed with nonrenal stromal cells and HSPCs. Putative HSC of the zebrafish have been found under normal conditions to be located at the surfaces of renal tubules,20 and putative HSCs of carp have also been reported to home to renal tubules shortly after transplantation.21 Although this implies that renal tubule cells form an important component of the adult HSC niche in teleosts, putative HSC niche cells have not been described in the adult zebrafish, nor is it known whether high sdf-1 expression is a characteristic of the zebrafish HSC niche.
Although mammals have a single sdf-1 gene with multiple splice variants, zebrafish have 2 sdf-1 genes, sdf-1a/(cxcl12a) and sdf-1b/(cxcl12b), which arose from a genome duplication event. sdf-1a and sdf-1b share approximately 45% and 47% nucleotide identity, respectively, with the human sdf-1 gene.22 The zebrafish sdf-1a and sdf-1b genes are located on different chromosomes and share approximately 75% amino acid identity.22,23 We report that the anatomic locations of sdf-1 expression in the zebrafish are similar to those patterns previously reported in mammals. Moreover, sdf-1 is expressed in high levels in renal tubule cells, which compose a majority of the marrow in the adult zebrafish and may form the HSPC niche. Sdf-1 is up-regulated in a dose-dependent fashion after radiation in the zebrafish and acts as a chemoattractant to adult zebrafish hematopoietic cells. Because previous studies22 as well as our own experiments indicated predominant expression of sdf-1a, rather than sdf-1b, in the teleost hematopoietic tissue specifically, we hypothesized that sdf-1a might play a predominant role in recruiting HSPCs. Therefore, we created a transgenic fluorescence reporter of sdf-1a expression to achieve simultaneous assessment of donor-derived hematopoietic cell migration and sdf-1a expression patterns in HSPC transplant recipients. Using this transgenic reporter, putative hematopoietic niche-defining cells in the kidney were localized as kidney tubule cells. Sdf-1a–expressing cells also were found in regions throughout the skin of the transplant recipients. Cumulatively, our findings suggest that SDF-1 may guide hematopoietic cell migration after HCT in the adult zebrafish to both medullary and extramedullary sites.
Methods
Zebrafish strains and fish husbandry
Fish were maintained by the University of Minnesota Zebrafish Core Facility according to standardized procedures24 and with the approval of the Institutional Animal Care and Use Committee, University of Minnesota. Wild-type fish were obtained from Segrest Farms and bred in-house. Other fish strains used include AB, Tg(atp1a1a.4:EGFP),25 Tg(fli1:EGFP),26 the Casper mutant,27 and the odysseus mutant.28
Transgenic construction
The 4.3-kb promoter region of sdf-1a was cloned upstream of the DsRed2 fluorophore in the Tol2 backbone and injected along with Tol2 transposase mRNA to facilitate integration, into early zebrafish embryos.29 Embryos were screened for fluorescence, raised to maturity, and bred to identify founders. Two independent transgenic lines, each generated from a separate founder fish, were used for this work.
Fish irradiation
Radiation was delivered through one of 2 systems
With a J.L. Shepherd Mark 1 Model 30 Irradiator, with a Cs137 source. Radiation chambers were constructed from Petri dishes radially divided with Plexiglas into 16 wedge-shaped compartments filled with fish water, each compartment containing a single unanesthetized zebrafish. Radiation chambers were placed on a rotating turntable in front of the source. Fish were irradiated with a single, unfractionated dose at a rate of 6.55 Gy/min. The mean lethal dose identified for this cesium system was 30 Gy, and this dose was used for cesium radiation preconditioning for most transplant experiments.
With a X-Rad320 irradiator (Precision X-Ray Inc). Unanesthetized fish were placed in a Petri dish filled with fish water and placed below the source. Fish were irradiated with a single, unfractionated dose at a rate of 2.7 Gy/min. The mean lethal dose identified for this x-ray system was 20 Gy, and this dose was used for x-ray preconditioning for most transplant experiments.
The studies were started with the cesium-based source, but this was decommissioned partway through the experiments. Subsequent studies were performed on the x-ray–based source, and there were no biologic differences found in the ability of the 2 radiation systems to up-regulate sdf-1 or myeloablate recipients. Kaplan-Meier survival graphs documenting survival after radiation doses were prepared for each of these radiation delivery methods (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Transplantion
Two days before transplantation, transplant recipients were preconditioned with sublethal radiation (specific dose depending on the radiation system as described in “Fish irradiation”). To isolate cells for HCT, donor kidneys were flushed and harvested into phosphate-buffered saline (PBS). Whole kidney marrow (WKM) was isolated by trituration, filtered through a 40-μm filter, and centrifuged at 2000g for 6 minutes. For transplant of odysseus WKM, vital dye labeling was performed in serum-free, growth-factor free Zebrafish Kidney Stromal Media30 using CellTracker Green CMFDA (Invitrogen), per the manufacturer's instructions. Supernatant was removed, cells were resuspended in PBS, counted, and kept on ice until transplant. A total of 280 000 dyed WKM cells were transplanted into each recipient via cardiac injections. For myelomonocyte transplants, cells were isolated from WKM via flow-sorting according to forward scatter/side scatter (FSC/SSC) characteristics, counted, and kept on ice before transplant. A total of 100 000 myelomonocytes were transplanted into each recipient.
In situ hybridization
Whole-mount in situ hybridization was performed by previously described methods.31 Tissue was harvested and fixed overnight at 4°C in 10% neutral buffered formalin, and dehydrated and stored in methanol at −20°C. Tissues were bleached in 1.5% H2O2 for 20 minutes and permeabilized with 10 μg/mL Proteinase K for 45 minutes at room temperature. Sense and antisense probes labeled with digoxigenin were applied at concentrations of 1 μg/mL in hybridization buffer, rotated overnight at 55°C, detected with anti-digoxigenin-AP Fab fragments, and developed with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (Roche Diagnostics) and 1mM levamisole. Signal in whole tissues was fixed in 4% paraformaldehyde, rinsed, cleared and photographed in glycerol, and then rinsed and soaked overnight at 4°C in 30% sucrose in PBS, sectioned, counterstained with Nuclear Fast Red (Vector Laboratories), dehydrated in ethanol, cleared in xylene, and mounted in DPX.
Quantitative RT-PCR
Zebrafish were killed in tricaine. Fish were beheaded and kidneys were flushed with PBS to remove a large amount of blood from the circulation. For preparation of separate tubule and nontubule fractions for quantitative reverse-transcribed polymerase chain reaction (qRT-PCR), kidneys were harvested into PBS on ice, triturated, and filtered through a 40-μm filter to collect small cells that passed through the filter. These cells were referred to as the nontubule fraction. Tissue failing to pass through the filter (kidney tubules and other stroma) was released from the inverted filter with PBS, and referred to as the kidney tubule fraction. Tubule and nontubule fractions were then centrifuged at 2000g for 5 minutes, PBS was removed, and pellets were frozen in liquid nitrogen. Skin samples were collected by isolating skin from muscle. Total RNA was isolated using Trizol Reagent (Invitrogen) according to guidelines of the manufacturer. cDNA was reverse-transcribed with Superscript III RT-PCR kit (Invitrogen) using random hexamers and quantitative RT-PCR was performed on an Applied Biosystems StepOnePlus, using SYBR Green reagent (Invitrogen). Intron-spanning primers were used: sdf-1a (5′cgccattcatgcaccgatttc and 5′ggtgggctgtcagatttccttgtc), sdf-1b (5′cgccttctggagcccagaga and 5′agagattctccgctgtcctcc), and β-actin (5′gatgcccctcgtgctgtttt and 5′tctctgttggctttgggattca). Relative quantitation (RQ) values were calculated from duplicate reactions for each sample using the δ-δ-Ct method.
In vitro migration assay
Kidneys from large adult wild-type (WT) zebrafish were flushed with PBS, dissected, treated for 5 minutes in 0.0001% bleach in PBS, rinsed several times in PBS, triturated, and filtered through 40-μm filter to isolate HSPC. Cells were centrifuged at 2000g for 6 minutes and resuspended in 1:1 Dulbecco modified Eagle medium:F12 (Invitrogen) plus 0.5% bovine serum albumin. Treatment of cells with AMD3100 (Sigma-Aldrich, #A5602) was performed with rotation at room temperature for 2 hours at a drug concentration of 660μM. Then, 3.4 to 3.5 × 106 whole kidney marrow cells in 200 μL were loaded into a Transwell filter basket (5-μm pore size, 6.5-mm insert, Costar). Each transwell basket was placed into a culture well containing 500 μL media to which human recombinant SDF-1 (hrSDF-1α; Calbiochem, #572300) had been added. Cells were allowed to migrate for 2 hours at 32°C. After removal of Transwell baskets, migrated cells in each culture well were manually counted by a hematocytometer and subjected to flow cytometry and a uniform flow rate and sample acquisition time period for SSC/FSC analysis.
Statistical analysis
Quantitative data were entered into Prism Version 5.0 (GraphPad Software). Transplant experiments comparing 2 groups were analyzed by unpaired 2-tailed t test. For experiments comparing more than 2 groups, analysis of variance analysis with Tukey postanalysis was performed, and P values less than .05 were identified as significant.
Imaging
Unless otherwise specified, photographs were obtained at room temperature using a DMI series inverted microscope (Leica), Retiga 2000R camera (Qimaging), and QCapture V2 acquisition software. Acquisition of brightfield images additionally used a QImaging RGB color filter. Live fish were imaged in water, and fresh isolated tissues were imaged in glycerol and PBS. In Adobe Photoshop CS3 and CS4, fluorescence images were pseudocolored according to the emission of the fluorophore, and pseudocolored photographs of multiple color channels of a single field of view were merged together. Specimens that exceeded a single field of view were documented in several epifluorescence photographs along their length on the x,y plane, and photo montages were assembled manually in Adobe Photoshop. Where sample thickness required it, epifluorescence z-stacks were acquired, processed for deconvolution using MetaMorph, and subsequently merged along the z-axis to produce a single image (see Figure 4F).
Results
sdf-1 expression patterns in adult zebrafish
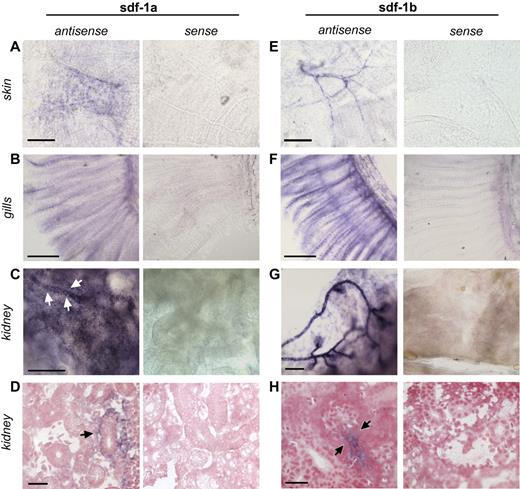
Because of the paucity of data about the anatomic expression patterns of sdf-1 in the adult zebrafish, whole-mount in situ hybridization (WISH) for sdf-1 expression studies were performed. There are 2 zebrafish sdf-1 genes, sdf-1a and sdf-1b, with unknown, if any, divergence in expression and hematopoietic function in adults. Basal sdf-1a and sdf-1b expression was seen in adult skin (Figure 1A,E), gills (Figure 1B,F), and kidney (Figure 1C-D,G-H). sdf-1a–expressing cells in the kidney, the site for hematopoiesis in the zebrafish, included renal epithelial tubular cells and a few vascular-endothelial cells (Figure 1C-D), as well as cells of unknown type interspersed between renal tubules (supplemental Figure 2). Sdf-1b expression appears strongest in renal tubules, and particularly adjacent to glomeruli (Figure 1G-H). Signal was not seen in controls stained with the sense probe. Thus, sdf-1a and sdf-1b expression in adult zebrafish encompasses both known sites of hematopoiesis (the kidney) as well as in selected nonhematopoietic organs (the skin and gills), consistent with the possibility that sdf-1 in adult zebrafish may regulate both medullary and extramedullary hematopoietic cell migration.
In situ hybridization of zebrafish tissues. Whole-mount in situ hybridization was performed on tissues from adult zebrafish skin (A,E), gills (B,F), and kidney (C-D,G-H). (A) sdf-1a expression in a chevron-shaped pattern in the skin. Bar represents 150 μm. (E) sdf-1b expression in skin structures with vascular morphology. Bar represents 150 μm. (B) sdf-1a expression in gills. Bar represents 250 μm. (F) sdf-1b expression in gills. Bar represents 250 μm. (C) sdf-1a expression in the kidney. Arrows indicate kidney tubule. Bar represents 100 μm. (D) Arrow indicates sdf-1a expression in renal tubules cells. Bar represents 25 μm. (G) sdf-1b expression in the kidney, showing predominant signal located in areas of glomeruli and adjacent tubules. Bar represents 100 μm. (H) Arrows indicate sdf-1b expression in proximal renal tubule. Bar represents 25 μm.
In situ hybridization of zebrafish tissues. Whole-mount in situ hybridization was performed on tissues from adult zebrafish skin (A,E), gills (B,F), and kidney (C-D,G-H). (A) sdf-1a expression in a chevron-shaped pattern in the skin. Bar represents 150 μm. (E) sdf-1b expression in skin structures with vascular morphology. Bar represents 150 μm. (B) sdf-1a expression in gills. Bar represents 250 μm. (F) sdf-1b expression in gills. Bar represents 250 μm. (C) sdf-1a expression in the kidney. Arrows indicate kidney tubule. Bar represents 100 μm. (D) Arrow indicates sdf-1a expression in renal tubules cells. Bar represents 25 μm. (G) sdf-1b expression in the kidney, showing predominant signal located in areas of glomeruli and adjacent tubules. Bar represents 100 μm. (H) Arrows indicate sdf-1b expression in proximal renal tubule. Bar represents 25 μm.
Radiation-induced up-regulation of sdf-1 in the hematopoietic cell niche of the zebrafish
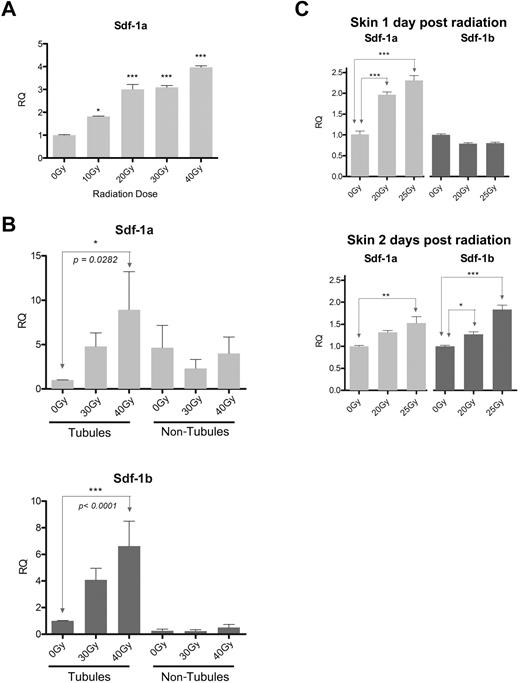
In mammals, radiation is known to up-regulate sdf-1 expression in the bone marrow compartment, which probably contributes to homing of HSCs and HSPCs to bone marrow.14 To determine whether sdf-1 expression is up-regulated in the zebrafish marrow in response to radiation, sdf-1 expression levels were assessed in the kidney after a range of radiation doses. One day after radiation, sdf-1a expression in the whole kidney in WT zebrafish increased by nearly 4-fold when analyzed after 40 Gy compared with nonirradiated controls; in addition, a dose-response effect was observed at lower radiation doses (Figure 2A).
sdf-1 up-regulation in kidney and skin in response to preconditioning radiation. (A) Quantitative RT-PCR of whole zebrafish kidneys 1 day after fish received Cs137 radiation. n = 5 adult fish per group. (B) Quantitative RT-PCR of isolated renal tubule fractions and nontubule fractions 1 day after fish received Cs137 radiation. Data represent pooled results from 2 independent biologic experiments, with an ultimate total of 20 to 24 fish per condition. (C) Quantitative RT-PCR of skin 1 day (above) and 2 days (below) after fish received x-ray irradiation; n = skin from 5 fish per condition. (A-C) Experimental primers were normalized to B-actin controls. Error bars show mean RQ and SEM between duplicate quantitative RT-PCR reactions. Each radiation group is compared with the 0-Gy reference. ***P < .001 (analysis of variance with Tukey postanalysis). **.001 < P < .01 (analysis of variance with Tukey postanalysis). *.01 < P < .05 (analysis of variance with Tukey postanalysis).
sdf-1 up-regulation in kidney and skin in response to preconditioning radiation. (A) Quantitative RT-PCR of whole zebrafish kidneys 1 day after fish received Cs137 radiation. n = 5 adult fish per group. (B) Quantitative RT-PCR of isolated renal tubule fractions and nontubule fractions 1 day after fish received Cs137 radiation. Data represent pooled results from 2 independent biologic experiments, with an ultimate total of 20 to 24 fish per condition. (C) Quantitative RT-PCR of skin 1 day (above) and 2 days (below) after fish received x-ray irradiation; n = skin from 5 fish per condition. (A-C) Experimental primers were normalized to B-actin controls. Error bars show mean RQ and SEM between duplicate quantitative RT-PCR reactions. Each radiation group is compared with the 0-Gy reference. ***P < .001 (analysis of variance with Tukey postanalysis). **.001 < P < .01 (analysis of variance with Tukey postanalysis). *.01 < P < .05 (analysis of variance with Tukey postanalysis).
Because radiation up-regulates sdf-1a in the kidney and sdf-1a shows expression both in renal tubules and in smaller nonrenal cells dispersed between the tubules, we next sought to determine whether the cells responsible for radiation-induced up-regulation of sdf-1 expression are located in the tubule fraction or located the nontubule (HSPC-containing) fraction of the kidney. As sdf-1 is expressed by the mammalian HSC niche and acts to attract and retain HSCs during hematopoiesis and during HCT, a renal tubule-specific up-regulation of sdf-1 in response to radiation would support the hypothesis that a zebrafish HSC niche is composed of tubule cells. Trituration and filtration crudely separate the kidney tissue into a tubule fraction enriched for renal tubules and blood vessels, and a fraction composed of smaller cells passing through the filter, which are mostly HSPCs. Although sdf-1a expression is present in the nontubule fraction, we find that radiation-induced increase in both sdf-1a and sdf-1b expression is most pronounced in the tubule fraction versus the nontubule fraction one day after radiation (Figure 2B).
Because sdf-1 expression has been reported to be up-regulated in response to injury in mammalian skin, promoting multipotent stem cell migration to injured sites,32 we hypothesized that radiation exposure could up-regulate sdf-1 expression in zebrafish skin as well. Quantitative RT-PCR indeed indicated that radiation-induced up-regulation of sdf-1 expression in the zebrafish is not limited to the kidney but also occurred in the skin after whole body irradiation of fish (Figure 2C). Both sdf-1a and sdf-1b were up-regulated after irradiation, although sdf-1a expression was up-regulated by day 1, whereas sdf-1b up-regulation was not evident until the second day after radiation in the skin. Cumulatively, these findings indicate that radiation causes sdf-1a and sdf-1b up-regulation in the adult zebrafish kidney, predominantly in the renal tubule cell fraction, as well as the skin.
SDF-1 is a chemoattractant for adult zebrafish hematopoietic cells
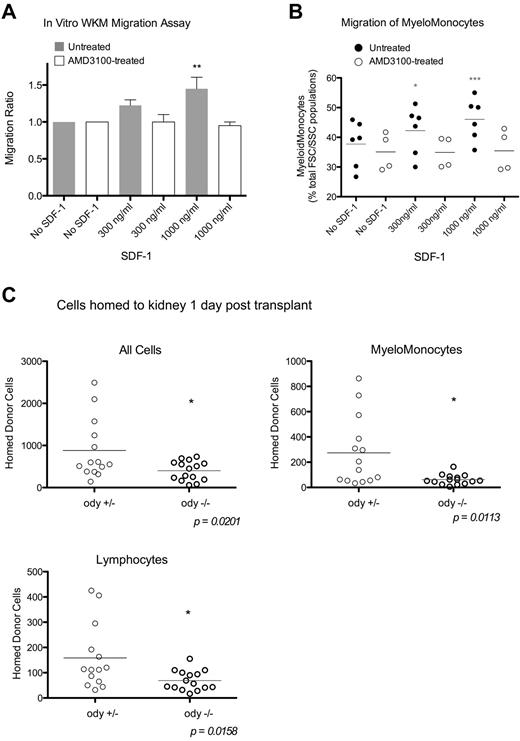
Having established that radiation increases sdf-1a and sdf-1b in the putative HSC niche of the zebrafish, we next sought to evaluate the capability of SDF-1 to induce migration of zebrafish hematopoietic cells. Therefore, in vitro migration assays were performed using whole kidney marrow, which contains HSCs and HPSCs, from WT zebrafish. Within 2 hours, the application of 1 μg/mL concentration of hrSDF-1a induced significant migration of WKM (Figure 3A). Flow cytometric analysis of migrated cells indicated that myelomonocytes were the predominant cell type migrating toward hrSDF-1 and that such migration occurred in an hrSDF-1 concentration-dependent fashion (Figure 3B). This migration was blocked by pretreatment of WKM cells with AMD3100, a chemical antagonist that blocks the CXCR4:SDF-1 interaction, used at a concentration of 660μM, the maximum concentration that did not increase apoptosis of WKM cells compared with nontreated controls. Migration of other cell populations (lymphocytes and precursors) showed no significant increase with the addition of hrSDF-1 (data not shown).
SDF-1 chemoattracts zebrafish WKM cells. (A) Two-hour in vitro transwell migration assay, with migration ratios calculated from hematocytometer total cell counts. Untreated, n = 4 experiments; and AMD3100-treated, n = 2 experiments. Error bars represent SEM. (B) Fluorescence-activated cell sorter analysis of migrated cells from in vitro migration assays. Percentage myelomonocytes are calculated from a cell population with FSC/SSC properties of lymphocytes, precursors, and myelomonocytes. Bars show the mean. Untreated, n = 4 experiments; and AMD3100-treated, n = 3 experiments. ***P < .001 (analysis of variance with Tukey postanalysis). **.001 < P < .01 (analysis of variance with Tukey postanalysis). *.01 < P < .05 (analysis of variance with Tukey postanalysis). (C) odysseus mutant WKM was labeled with vital dye and transplanted into AB and WT fish preconditioned with 20 Gy x-ray irradiation. Recipient kidneys were harvested and analyzed by flow cytometry 1 day after transplantation. Graphs represent the number of donor-derived cells detected per 60 000 total cells. Bars represent the mean; n = 2 experiments, unpaired t test.
SDF-1 chemoattracts zebrafish WKM cells. (A) Two-hour in vitro transwell migration assay, with migration ratios calculated from hematocytometer total cell counts. Untreated, n = 4 experiments; and AMD3100-treated, n = 2 experiments. Error bars represent SEM. (B) Fluorescence-activated cell sorter analysis of migrated cells from in vitro migration assays. Percentage myelomonocytes are calculated from a cell population with FSC/SSC properties of lymphocytes, precursors, and myelomonocytes. Bars show the mean. Untreated, n = 4 experiments; and AMD3100-treated, n = 3 experiments. ***P < .001 (analysis of variance with Tukey postanalysis). **.001 < P < .01 (analysis of variance with Tukey postanalysis). *.01 < P < .05 (analysis of variance with Tukey postanalysis). (C) odysseus mutant WKM was labeled with vital dye and transplanted into AB and WT fish preconditioned with 20 Gy x-ray irradiation. Recipient kidneys were harvested and analyzed by flow cytometry 1 day after transplantation. Graphs represent the number of donor-derived cells detected per 60 000 total cells. Bars represent the mean; n = 2 experiments, unpaired t test.
These in vitro studies showing that SDF-1 induces migration of zebrafish hematopoietic cells, combined with our finding of radiation-induced sdf-1 up-regulation in hematopoietic tissue, suggested that endogenous sdf-1 directs hematopoietic cell homing in vivo in irradiated adult zebrafish during HCT. To determine whether in vivo administration of AMD3100 could be used to inhibit HSPC homing similar to the in vitro effect we observed, we performed HCT with WKM cells pretreated for 2 hours with 660μM AMD3100. In 3 independent trials, impairment of homing was subtle and subsequent experiments with different cell doses and different genetic backgrounds failed to confirm these initial results (data not shown).
In other studies, we attempted to use AMD3100 as a mobilizing agent. We found no changes in HSPC mobilization in fish treated with AMD3100 (supplemental Figure 3). Future experiments examining different doses and schedules of AMD3100 would be required to reach a conclusion as to whether AMD3100 has the capacity in zebrafish to block Sdf-1:Cxcr4 binding in vivo.
To further assess the possibility that Sdf-1:Cxcr4 interaction regulates the homing of WKM cells in vivo, we took advantage of a zebrafish line, odysseus, in which 1 of the 2 cxcr4 genes is mutated (cxcr4b specifically).28 We performed HCT using donor hematopoietic (WKM) cells obtained from the adult odysseus and found that transplants of homozygous odysseus mutant hematopoietic cells resulted in significantly fewer homed cells in the WT recipient kidney than transplants of heterozygous mutant hematopoietic cells (Figure 3C). The homing of lymphoid and, to a greater extent, myelomonocytic lineage cells were reduced when homozygous odysseus versus heterozygous WKM cells were infused. These data indicated that the Sdf-1/Cxcr4 axis is important in zebrafish hematopoietic cell homing as is the case in mammals.
To confirm sdf-1 receptor expression in WKM cells, CXCR4 immunofluorescence staining of WKM populations sorted according to FSC/SSC properties was performed. Cells expressing CXCR4 were present in all 3 cell populations. Qualitatively, the greatest prevalence of CXCR4-positive cells resided in the myelomonocyte population (supplemental Figure 4) as would be predicted by the results of the in vitro WKM migration assay.
Localization of sdf-1a expression in adult transgenic zebrafish
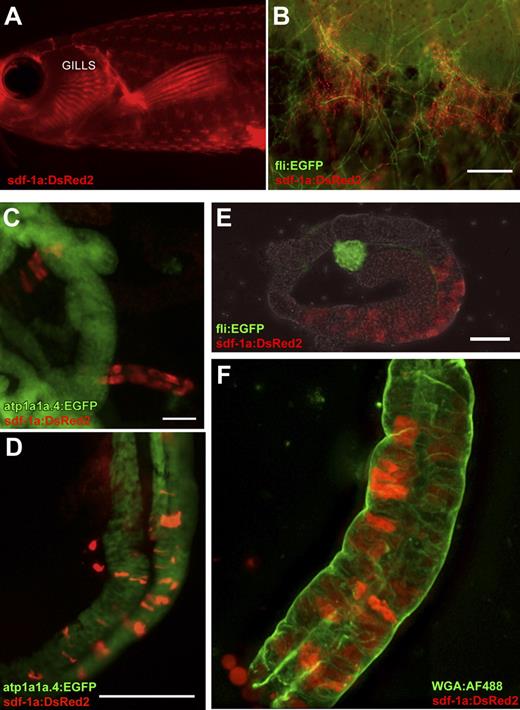
Quantitative RT-PCR analysis of WT zebrafish organs indicated greater constitutive expression of sdf-1a in the adult zebrafish kidney than sdf-1b (supplemental Figure 5). To more precisely analyze cells that define the putative hematopoietic cell niche in zebrafish, we decided to generate sdf-1a transgenic zebrafish that contained the sdf-1a promoter linked to a reporter fluorophore, DsRed2. The resulting transgenic line permits in vivo fluorescent detection of sdf-1a promoter activity, which mirrors that of the endogenous promoter. For this purpose, the 4.3-kb promoter region of sdf-1a was cloned upstream of the DsRed2 fluorophore in the Tol2 backbone and injected into early zebrafish embryos along with Tol2 transposase mRNA to facilitate integration. Two independent lines of Tg(sdf-1a:DsRed2) with similar expression patterns were used, each generated from a different founder fish. The use of Tg(sdf-1a:DsRed2) zebrafish avoids the technical issues of fixation and processing that is required for WISH and also facilitates flow cytometry–based analysis and isolation. In the Tg(sdf-1a:DsRed2), sdf-1a expression in the intact organism is readily visualized in the skin in chevron-shaped structures and gills (Figure 4A-B) as well as in renal tubules (Figure 4C-F), mirroring the mRNA expression patterns observed by WISH.
sdf-1a expression reported by Tg(sdf-1a:DsRed2). (A) In vivo fluorescence signal reporting sdf-1a expression in the adult transgenic, revealing expression in the skin and gills (imaged with a Leica MZ FLIII fluorescence stereomicroscope). (B) In vivo sdf-1a:DsRed2 (red) indicates sdf-1a expression in chevron-shaped structures in the skin, and fli:EGFP (green) expression marks vessels in a double transgenic fish with both reporters. Bar represents 200 μm. (C) sdf-1a:DsRed2 expression in renal tubules that do not report expression of atp1a1a.4, in a fish transgenic for both reporters. Bar represents 50 μm. (D) sdf-1a:DsRed2 expression in distal renal tubules that also express atp1a1a.4, in a fish transgenic for both reporters. Bar represents 125 μm. (E) fli:EGFP expression marking vessels of a glomerulus, and sdf-1aDsRed2 expression in an immediately adjacent (proximal) renal tubule of a transgenic fish with both reporters, imaged in PBS. Bar represents 50 μm. (F) An isolated kidney tubule showing sdf-1a:DsRed2 signal in renal tubule cells. Green represents WGA-AF488 applied to fresh tubules at 10 μg/mL for 20 minutes on ice (a concentration calculated to produce widespread nonspecific labeling for purposes of revealing basic tubule morphology). After staining, specimen was rinsed in PBS and immediately imaged in glycerol compressed between 2 glass coverslips, using a 40× oil-immersion objective.
sdf-1a expression reported by Tg(sdf-1a:DsRed2). (A) In vivo fluorescence signal reporting sdf-1a expression in the adult transgenic, revealing expression in the skin and gills (imaged with a Leica MZ FLIII fluorescence stereomicroscope). (B) In vivo sdf-1a:DsRed2 (red) indicates sdf-1a expression in chevron-shaped structures in the skin, and fli:EGFP (green) expression marks vessels in a double transgenic fish with both reporters. Bar represents 200 μm. (C) sdf-1a:DsRed2 expression in renal tubules that do not report expression of atp1a1a.4, in a fish transgenic for both reporters. Bar represents 50 μm. (D) sdf-1a:DsRed2 expression in distal renal tubules that also express atp1a1a.4, in a fish transgenic for both reporters. Bar represents 125 μm. (E) fli:EGFP expression marking vessels of a glomerulus, and sdf-1aDsRed2 expression in an immediately adjacent (proximal) renal tubule of a transgenic fish with both reporters, imaged in PBS. Bar represents 50 μm. (F) An isolated kidney tubule showing sdf-1a:DsRed2 signal in renal tubule cells. Green represents WGA-AF488 applied to fresh tubules at 10 μg/mL for 20 minutes on ice (a concentration calculated to produce widespread nonspecific labeling for purposes of revealing basic tubule morphology). After staining, specimen was rinsed in PBS and immediately imaged in glycerol compressed between 2 glass coverslips, using a 40× oil-immersion objective.
To better define the relationship between sdf-1a expression and blood vessels, the Tg(sdf-1:Dsred2) was mated with a fli-1:EGFP transgenic zebrafish. The transcription factor fli-1 is expressed in all vessels; hence, the blood vasculature in fli-1:EGFP transgenics is GFP-positive. We find that chevron-shaped sites of sdf-1a expression in the skin exist almost exclusively in regions of high concentrations of fli-1+ blood vessels, which form islands of vascular plexuses distributed across the body of the fish in a pattern that mirrors the distribution of the fish scales (Figure 4A-B).
To clarify the nature of the tubules containing cells expressing sdf-1a, Tg(sdf-1a:DsRed2), was crossed to Tg(atp1a1a.4:EGFP), which labels distal tubules and collecting ducts within the adult kidney (Iain Drummond, personal communication, August 3, 2009). Examination of the double transgenic adult zebrafish kidney revealed colocalization of sdf-1a and atp1a1a.4-negative renal tubules (Figure 4C), as well sdf-1a expression in atp1a1a.4:EGFP-positive renal tubules (Figure 4D). sdf-1a:DsRed2-expressing kidney tubules are also found located adjacent to glomeruli (Figure 4E). Taken together, these data indicate that cells with high sdf-1a expression are present in proximal tubules as well as distal tubules or collecting ducts.
Hematopoietic cells migrate in vivo toward sites of sdf-1 expression in zebrafish after radiation and HCT
One day after 40 or 50 Gy irradiation, adult Tg(sdf-1a:DsRed2) showed increased numbers of DsRed2-positive renal tubule cells, as assessed by flow cytometric analysis (supplemental Figure 6). There was no difference in the mean fluorescence intensity of these cells indicating that radiation did not change the amount of DsRed protein expressed in renal tubule cells but rather increased the frequency of cells that expressed sdf-1a (numbers of cells are determined by percentage positive × total cells; MFI indicates expression levels per cell if gating is only on positive cells.)
Tg(sdf-1a:DsRed2) were then assessed by microscopy to determine whether transplanted hematopoietic cells homed to regions of high sdf-1a expression in the zebrafish in the context of HCT. Three days after WKM transplants in irradiated recipients, GFP-positive donor-derived cells can be found localized to the regions of the skin that showed DsRed2 expression (Figure 5B). Two days after transplanting a fraction of WKM cells enriched for the myelomonocyte lineage, fluorescent donor-derived cells were found distributed throughout the kidney, with some transplanted cells found immediately adjacent to tubule cells expressing dsRed (Figure 5A). Analysis of 3 recipient fish in the same experiment indicated donor-derived cells were localized with a significantly higher frequency to regions of sdf-1a:DsRed signal in the recipient kidney than would be expected of random distribution (supplemental Figure 7). Consistent with in vitro data and transplant experiments demonstrating the migration of hematopoietic cells to sdf-1, exposure of the zebrafish to radiation up-regulated sdf-1 in the adult zebrafish kidney, the putative HSC niche, and hematopoietic cells indeed migrated to sites of high sdf-1a expression in the kidney and skin.
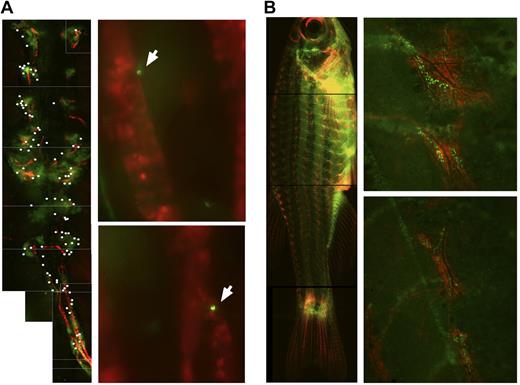
After HCT, donor cells migrate to sdf-1a:DsRed2 structures in recipients. (A) Photo composite of images taken with a 10×/0.3 NA objective of an ex vivo kidney of Tg(sdf-1a:DsRed2) 4 days after preconditioning with 30 Gy cesium radiation and 2 days after transplant with 100 000 Green Glofish MyeloMonocyte cells. For clarity, locations of donor-derived GFP+ cells are marked with a white dot (left panel). GFP+ donor-derived cells can be found adjacent to sdf-1a:DsRed2+ tubules. Photos taken with a 40×/0.6 NA objective (right panels). (B) Photo composite of a Tg(sdf-1a:DsRed2) fish 5 days after preconditioning with 25 Gy x-ray radiation and 3 days after transplantation with 1 × 106 Green Glofish WKM cells. Photos taken with a Leica M165 FC fluorescent microscope, Planapo 1.6×/.05 NA objective, Leica DFC340 FX camera, and Leica Application Suite software (left), and detail of regions of concentrated localizations of sdf-1a:dsRed cells and GFP+ donor-derived cells. Photos taken with a 10×/0.3 NA objective and IP Lab V4.0.8 acquisition software (right).
After HCT, donor cells migrate to sdf-1a:DsRed2 structures in recipients. (A) Photo composite of images taken with a 10×/0.3 NA objective of an ex vivo kidney of Tg(sdf-1a:DsRed2) 4 days after preconditioning with 30 Gy cesium radiation and 2 days after transplant with 100 000 Green Glofish MyeloMonocyte cells. For clarity, locations of donor-derived GFP+ cells are marked with a white dot (left panel). GFP+ donor-derived cells can be found adjacent to sdf-1a:DsRed2+ tubules. Photos taken with a 40×/0.6 NA objective (right panels). (B) Photo composite of a Tg(sdf-1a:DsRed2) fish 5 days after preconditioning with 25 Gy x-ray radiation and 3 days after transplantation with 1 × 106 Green Glofish WKM cells. Photos taken with a Leica M165 FC fluorescent microscope, Planapo 1.6×/.05 NA objective, Leica DFC340 FX camera, and Leica Application Suite software (left), and detail of regions of concentrated localizations of sdf-1a:dsRed cells and GFP+ donor-derived cells. Photos taken with a 10×/0.3 NA objective and IP Lab V4.0.8 acquisition software (right).
Discussion
SDF-1 orchestrates several hematopoietic cell migration and retention events in mammals, both during homeostasis as well as during HCT and hematopoietic reconstitution. It is a chemoattractant for several hematopoietic cell types, including HSPCs, leukocytes, and granulocytes, up-regulated in response to radiation-induced tissue damage, expressed in several locations throughout the organism, and present at high levels in the HSC niche in the bone marrow.33 Our data indicate that sdf-1 in the adult zebrafish has functional similarities to mammals in its up-regulation in response to radiation, capacity to induce hematopoietic cell migration, anatomic expression patterns in extramedullary sites throughout the organism, and high expression levels in putative HSC niche cells, which in the zebrafish include kidney tubular epithelial cells. Together, these data provide support for the use of zebrafish to study HSC and HSPC migration after radiation and HCT, allowing visualization of the homing pattern in the whole organism.
In the adult zebrafish, reagents for refined hematopoietic identification of specific cell populations, such as HSCs, are still in development. Nonetheless, our findings that zebrafish cells of myeloid characteristics show significant in vitro migration toward SDF-1, which can be inhibited by an AMD3100 blockade of Cxcr4, bear similarity to previous reports of migration of mammalian hematopoietic and effector cells toward SDF-1.34,35 This indicates that the capacity of sdf-1 to induce hematopoietic cell migration is conserved in the adult zebrafish. Although we were unable to document an effect of AMD3100 administration in vivo on homing of WKM cells during HCT or mobilization of WKM cells in nontransplanted zebrafish, which we hypothesize is the result of the lack of information as to the optimal dose and schedule of the drug, our observation that donor-derived WKM cells and myelomonocytes colocalize with sdf-1a:DsRed-positive cells in the skin and kidney after HCT is compatible with the conclusion that endogenous Sdf-1 acts as a bona fide hematopoietic chemoattractant in the zebrafish in vivo during HCT.
Data generated using WISH, quantitative RT-PCR, and the Tg(sdf-1a:DsRed2) reporter line indicate that some renal tubule cells express high levels of sdf-1. Furthermore, radiation-induced up-regulation of sdf-1 in the hematopoietic organ occurs in kidney tissue fractions enriched for renal tubules. Investigators have proposed that radiation-induced sdf-1 up-regulation specifically in “niche” cells of the bone marrow induces HSC homing to the niche after HCT.14,36 Previous suggestions in the literature indicate that the putative HSC niche of the zebrafish may be composed of renal tubule cells.20,21 These reports, combined with our findings here that renal tubule cells express high levels of sdf-1 and up-regulate expression in response to radiation, cumulatively indicate that sdf-1 may be a hallmark of a zebrafish HSC niche located on the renal tubules.
Further, our findings regarding sdf-1 expression in the whole organism have implications for extramedullary HSPC migration in mammals. Although SDF-1 expression has been reported previously in the human skin,8 our observation of WKM migration to concentrated regions of constitutively expressed sdf-1 in the zebrafish skin suggests the possibility of a dermal or epidermal structure that could act as an attractant or niche for stem or progenitor cells. Alternatively, CXCR4 expressing cells recruited to the skin may not be indicative of extramedullary hematopoiesis but instead may play a role in controlling inflammatory responses in the skin that occur after whole body irradiation.
Although the mature mammalian human kidney is not generally regarded to be a hematopoietic organ, it has been reported capable of supporting hematopoiesis.37 The presence of a renal tubule HSPC niche in the zebrafish raises the intriguing question of whether mammalian renal tubule cells retain some functions in stem cell attraction and maintenance. The fact that renal tubule expression of SDF-1 has also been reported in humans, mice, and rats suggests a conservation of useful biologic functions carried out by SDF-1 at this location. Indeed, SDF-1 expression by renal tubules has been strongly implicated in inducing cell migration and repair processes in the context of renal injury.38-40 Conversely, there are several reported characteristics of the mammalian HSC niche, such as high levels of SCF and ALCAM expression,4,16 as well as expression of adrenergic receptors and potential participation in neuroreticular complexes,13 which have yet to be characterized in this putative HSC niche of adult zebrafish. It remains to be seen whether the HSC niche in the adult zebrafish kidney shows similarity to the mammalian HSC niche with regards to broader gene expression profiles and physical proximity to other cell types or complexes that might contribute to HSC maintenance.
In conclusion, we have generated a sdf-1a:DsRed2 transgenic zebrafish that facilitates the identification of the HSC niche, probably renal tubule cells in the adult zebrafish. Moreover, our results indicate that, in the adult zebrafish, sdf-1 has similar roles in HSC attraction and support as it does in mammals. Although conclusive functional evidence of this will await the refinement of methods for the identification and isolation of HSCs in the adult zebrafish, the newly generated sdf-1a:Dsred2 transgenic line will permit flow cytometry isolation of putative cells in the HSC niche that may recruit donor HSPCs and support zebrafish hematopoiesis in the resting as well as perturbed state that accompanies radiation and HCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the University of Minnesota Zebrafish Core Facility for fish maintenance and breeding assistance, the Flow Cytometry Core Facility of the Masonic Cancer Center for assistance in cell sorting, Iain Drummond for providing the Tg(atp1a1a.4:EGFP) fish and for valuable discussion and insight, Jamie Van Berkum and Todd Smith for microscopy assistance, and Paul Stadem for assistance in sample preparations.
This work was supported by the Children's Cancer Research Fund and the 3M Corporation (graduate student fellowship) (T.J.G.).
Authorship
Contribution: T.J.G. and T.C.L. performed the experiments, analyzed results, and prepared the manuscript; X.P. assisted in experiments; and J.T., T.V.B., L.I.Z., and B.R.B. discussed experiments and results and edited the paper.
Conflict-of-interest disclosure: L.I.Z. is a founder and stockholder of Fate, Inc and a scientific advisor for Stemgent. The remaining authors declare no competing financial interests.
Correspondence: Troy C. Lund, MMC 109, University of Minnesota, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: lundx072@umn.edu.
References
Author notes
T.J.G. and T.C.L. contributed equally to this study.